Figures & data
Figure 1. Kinetics of CD8+ T cell responses to antigen stimulation. Wild type (WT) and B7-H1-deficient (KO) mice were immunized (i.p.) with OVA plus poly I:C. Kb/OVA tetramer was used to identify antigen-specific CD8+ T cells in spleen and liver at the indicated times after immunization. Data show the percentage of tetramer+ CD8+ T cells (mean ± SD of three mice per time point). One of two independent experiments is shown. * p < 0.05 compared with WT mice.
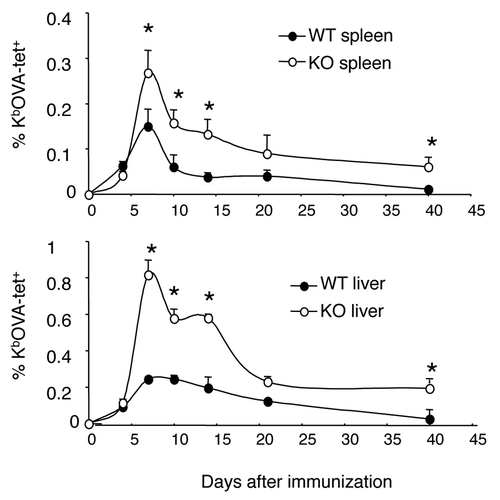
Figure 2. Enhanced memory CD8+ T-cell population in the absence of B7-H1. Mice were immunized with OVA plus poly I:C and were re-stimulated with OVA on day 40 after immunization. On day 4 after re-stimulation, spleen cells were isolated from naïve or immunized WT and B7-H1-deficient mice for analysis. (A) Percentage of OVA-specific tetramer+ CD8+ T cells, *p < 0.05 compared with WT mice. (B) Graph shows absolute number of OVA-specific tetramer+ CD8+ T cells (mean ± SD, n = 3). (C) Flow cytometry analysis of intracellular production of cytokines in CD8+ T cells from immunized mice (mean ± SD, n = 3). (D) In vivo cytolytic activity in immunized mice. OVA-peptide or control-peptide pulsed target cells (syngeneic splenocytes) were labeled with high or low dose CFSE (5 μM for OVA-peptide pulsed cells; 0.5 μM for control-peptide pulsed cells) and mixed (1:1, 2.5x106 of each) and injected i.v. into WT or B7-H1-deficient mice. Histogram plots show the percentage of remaining target cells in the spleen 4 h after target cell transfer. Bar graph shows percentage of specific lysis in the spleen (mean ± SD, n = 3).
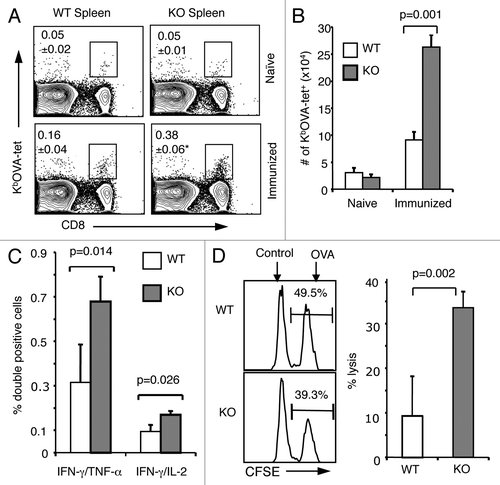
Figure 3. Enhanced memory CD8+ T-cell recall responses and improved antitumor immunity in the lung in the absence of B7-H1. On day 35 after immunization, immunized or naïve WT and B7-H1-deficient mice were injected (i.v.) with 5x105 B16-OVA tumor cells. (A) Percentage and absolute numbers of IFNγ+ CD8+ T cells in the lung of immunized mice (mean ± SD, n = 3) on day 4 after tumor injection. *p < 0.01 compared with WT mice. (B) Metastatic tumor foci in the lung tissue were identified and counted on day 20 after tumor injection (mean ± SD, n = 5). One of two independent experiments is shown. N.S.: not significant.
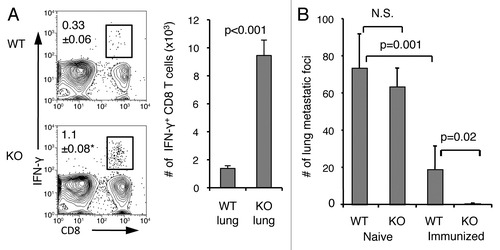
Figure 4. CD11ahigh CD8+ T cells represent antigen-primed effector T cells. Spleen cells from naïve or immunized WT and B7-H1-deficient mice were analyzed by co-staining with anti-CD11a and Kb/OVA tetramer or functional markers. (A) Percentage of CD11ahigh CD8+ T cells from WT and B7-H1-deficient immunized mice. (B) Graph shows average percentage of CD11ahigh CD8+ T cells from WT and B7-H1-deficient immunized mice (mean ± SD, n = 4). (C) Percentage of antigen-specific tetramer+ (Kb/OVA-tet) cells in CD11ahigh and CD11alow CD8+ T cell population. (D) CTL functional assay of CD11ahigh and CD11alow CD8+ T cells after a brief re-stimulation in vitro. Degranulation of CTLs was analyzed by CD107a mobilization, followed by intracellular staining for IFN-γ. Numbers indicate percentages of gated areas. One of three independent experiments is shown.
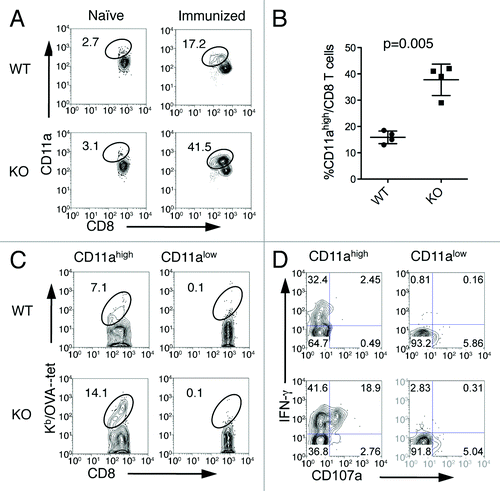
Figure 5. Fewer apoptotic antigen-primed CD8+ T cells in B7-H1-deficient mice. On day 7 after immunization, spleen cells were analyzed for proliferation and apoptosis. (A) Ki67 expression and (B) BrdU incorporation were analyzed in CD11ahigh or CD11alow CD8+ T cells. Numbers are percentages of gated area in total CD8+ T cells. (C) TMRElow Annexin V+ apoptotic cells were measured in CD11ahigh and CD11alow CD8+ T cells. (D) Graph shows percentage of apoptotic cells (TMRElow Annexin V+) in CD11ahigh CD8+ T cells (mean ± SD, n = 4). One of three experiments is shown.
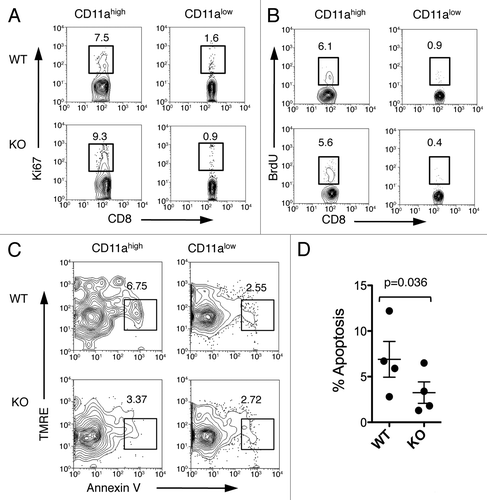
Figure 6. Lower Bim levels in antigen-primed CD8+ T cells in B7-H1-deficient mice. (A) Flow cytometry assay of the intracellular expression of Bim, Bcl-2 and Bcl-xL in gated CD11ahigh CD8+ T cells in the spleen of WT (red) and B7-H1-deficient (blue) mice on day 7 after immunization. Numbers are mean fluorescence intensity (MFI) of Bim expression. (B) Graph shows average MFI of Bim expressed by CD11ahigh CD8+ T cells (mean ± SD, n = 9). (C) Intracellular expression of Bim in CD11ahigh CD8+ T cells in the liver of immunized mice. Numbers are MFI. (D) Bim expression in total CD8+ T cells in the spleen of naive WT (red) and B7-H1-deficient (blue) mice. One of three experiments is shown.
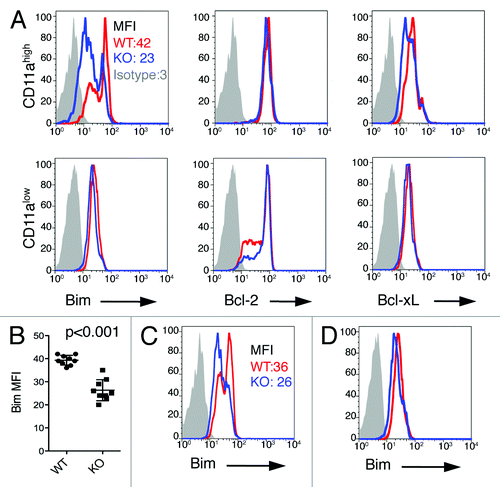
Figure 7. Extrinsic role of B7-H1 in regulation of Bim. WT OT-1 CD8+ T cells (Thy1.1+) were transferred in WT (red) or B7-H1-deficient (blue) host mice one day before immunization with OVA plus poly I:C. On day 7 after immunization, the OT-1 CD8+ T cells in the spleen and liver were identified by the Thy1.1 marker and analyzed for intracellular expression of Bim. Numbers are mean fluorescent intensity. One of two experiments is shown.
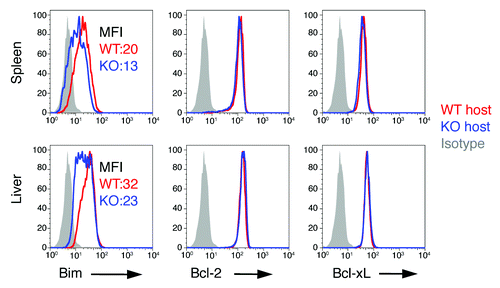
Figure 8. B7-H1 co-stimulation induces upregulation of Bim protein levels in activated T cells. Pre-activated CD8+ T cells were incubated with plate-bound B7-H1 or control fusion protein (Fc) for 48 h in the presence of anti-CD3. (A) Bim isoform expression in CD8+ T cells was analyzed by western blot. (B) Histogram shows the expression of total Bim in CD8+ T cells co-stimulated with B7-H1 (blue line) or control protein (red line). Numbers are mean fluorescent intensity (MFI) (C) Graph shows average MFI of Bim expressed by activated CD8+ T cells (mean ± SD, n = 5). (D) Graph shows the percentage of live (trypan blue exclusive) CD8+ T cells in culture (mean ± SD, n = 5). (E) Apoptosis of CD8+ T cells isolated from WT, Bim-deficient and Bcl-2 transgenic (Tg) mice. Numbers show percentage of TMRElow Annexin V+ apoptotic T cells in total CD8+ T cells. One of three experiments is shown. (F) Graph shows average MFI of Bim expressed by CD8+ T cells in culture with anti-B7-H1 Ab (10B5, blocking B7-H1 binding to both PD-1 and CD80; 43H12, blocking B7-H1 binding to CD80 only), anti-PD-1 Ab (G4) or control Ab (10 μg/mL of each) (mean ± SD, n = 3).
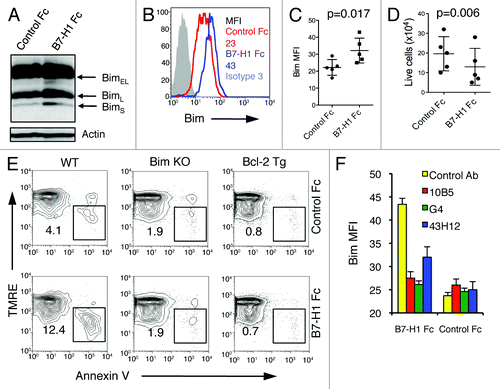
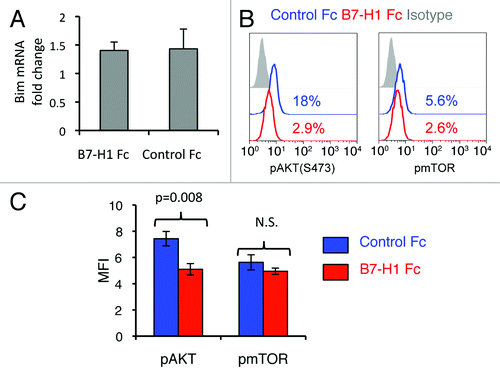
Figure 9. B7-H1 co-stimulation inhibits activation of Akt. Pre-activated CD8+ T cells were stimulated with plate-bound B7-H1 or control fusion protein (Fc). After 24 h of stimulation, CD8+ T cells were harvested and used for analysis. (A) Analysis of Bcl2l11 transcript levels by real-time qPCR using the comparative CT method. GAPDH served as the internal control gene. Graph shows fold change (mean ± SD, n = 4). (B) Phosphorylation of Akt and mTOR was analyzed by intracellular staining of CD8+ T cells with anti-phospho-Akt and anti-phospho-mTOR Abs. Numbers show percentage of positive stained cells. (C) Bar graph of average MFI of phospho-Akt and phospho-mTOR expression (mean ± SD, n = 3). N.S.: not significant.