Figures & data
Figure 1. Successful immunosurveillance of B-cell cancer in mice consists of an inflammatory reaction driven by tumor-specific Th1 cells. Eradication of myeloma and lymphoma in mice is achieved through a collaboration between tumor-specific Th1 cells and tumor-infiltrating, antigen-presenting M1 macrophages. During this process, nine cytokines are secreted locally by immune cells, including pro-inflammatory (IL-1α, IL-1β and IL-6) and Th1-associated cytokines (IL-12 and IFNγ). Th1 cells induce secretion of IL-1α, IL-1β, IL-6, CXCL-9 and CXCL-10 by inflammatory M1 macrophages. Th1-derived IFNγ renders macrophages directly cytotoxic to cancer cells. (Figure modified from ref. Citation19).
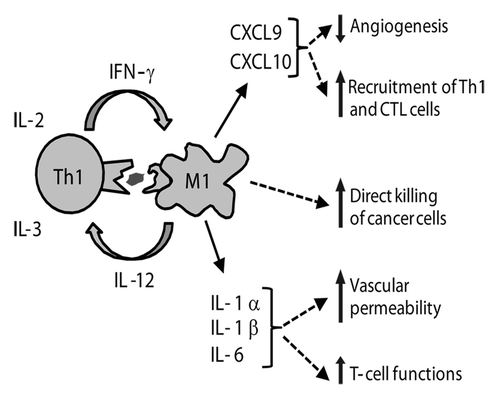
Figure 2. Cancer-suppressive properties of IL-1α and IL-1β. IL-1α and IL-1β synergize with IL-2 to stimulate the proliferation of CD4+ T cells, CD8+ T cells and NK cells; enhance the expansion and differentiation of CD4+ T cells; stimulate B-cell proliferation, generation of plasma cells and antibody production; induce the expression of adhesion molecules on vascular endothelium, which promotes extravasation of leukocytes into the inflamed tissue; directly inhibit the proliferation of specific cancer cells; render human macrophages cytotoxic to some cancer cells; and they amplify antigen-presenting functions and cytokine production by dendritic cells. Ab, antibody; APC, antigen-presenting cell; B, B cell; CD4, CD4+ T cell; CD8, CD8+ T cell; DC, dendritic cell; EC, endothelial cell; MΦ, macrophage; NK, natural killer cell.
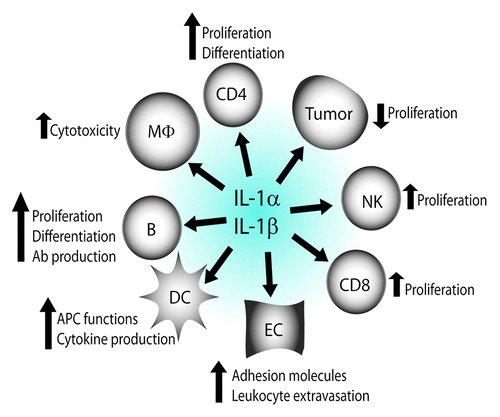
Figure 3. IL-1RI and TLRs share a common intracellular signaling pathway. Mature IL-1α and IL-1β mediate their functions by binding to the same IL-1 receptor I (IL-1RI) which is present on the surface of most cells (1a). Pathogen-associated molecular patterns (PAMP) and damage/danger-associated molecular patterns (DAMP) bind to Toll-like receptors (TLRs) (1b). IL-1RI and TLRs share a common intracellular signaling pathway (2), which leads to activation of NFκB and hence to the production of pro-inflammatory cytokines, such as IL-1α, IL-1β, IL-6 and TNFα (3). Therefore, IL-1α and IL-1β function within positive feedback loops in inflammation. Because of this common signaling pathway, IL-1α and IL-1β operate as natural adjuvants, mimicking the detection of microbial products by TLRs. MyD88, Myeloid Differentiation primary response gene 88; TRAF6, TNF receptor associated factor 6.
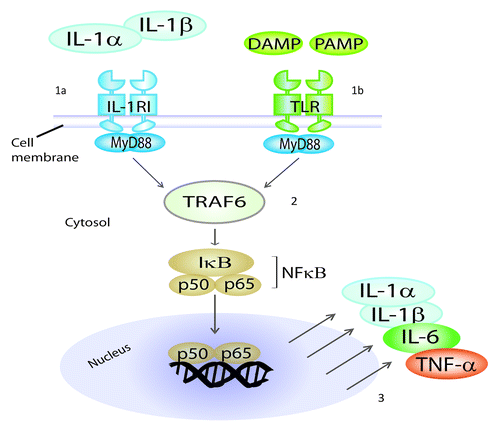
Figure 4. Cancer-suppressive properties of IL-6. IL-6 stimulates B-cell proliferation, differentiation as well as antibody production and it influences macrophage differentiation and activation. Treatment of mice with IL-6 may induce regression of established micrometastases in liver and lungs in a process which requires both CD4+ and CD8+ T cells. Immunization of mice with IL-6-transfected carcinoma cells may generate high levels of tumor-specific CD8+ T cells and functions as an efficient prophylactic and therapeutic cancer vaccine. Fibrosarcoma cells transduced with IL-6 exhibit reduced tumorigenicity, increased immunogenicity and decreased metastatic potential. IL-6 may increase CD8+ T cell trafficking to tumors by affecting endothelial cells and it inhibits proliferation of human acute myeloid leukemia, B-chronic lymphocytic leukemia and early-stage melanoma cells.
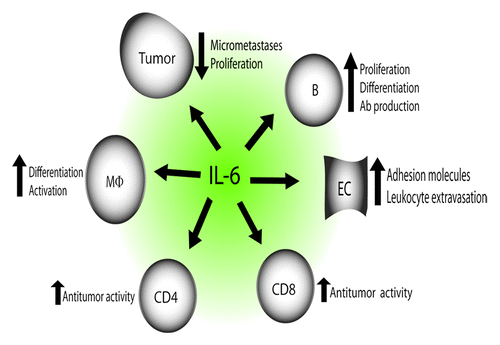
Figure 5. Cancer-suppressive properties of TNFα. TNFα stimulates T-cell activation, proliferation and recruitment; causes hemorrhagic necrosis of solid tumors through destruction of tumor blood vessels; is directly cytotoxic to several human cancer cell lines; and it renders human macrophages cytotoxic to some cancer cells. TNFα-transfected myeloma cells are eliminated in mice through recruitment and activation of macrophages. TNFα mediates recruitment of NK cells to the peritoneum to eliminate MHC Class I-negative cancer cells; is important for priming, proliferation and recruitment of tumor-specific CD8+ T cells; stimulates antigen-presenting cell functions and cytokine production; and it increases vascular permeability leading to improved penetration of chemotherapeutics in the tumor tissue.
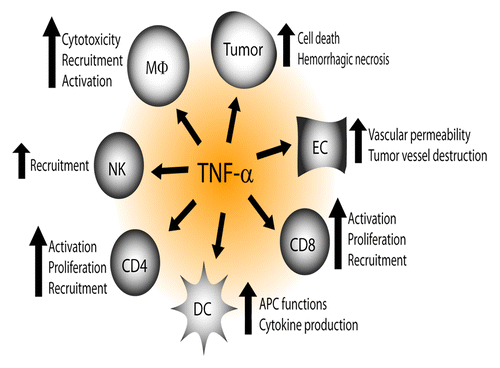
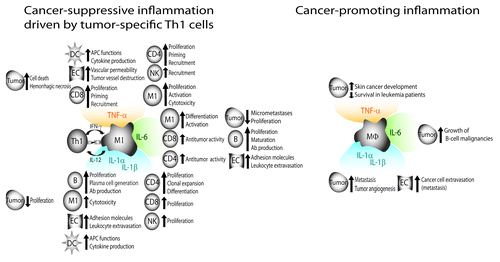
Figure 6. A model for how inflammation may either suppress or promote cancer. (Left) Cancer-suppressive inflammation driven by tumor-specific Th1 cells. Tumor-specific Th1 cells collaborate with tumor-infiltrating M1 macrophages to efficiently recognize and eliminate malignant cells. M1 macrophages function as efficient antigen-presenting cells that process and present cancer antigens to tumor-specific CD4+ and CD8+ T cells in the tumor. Th1 cells are characterized by the production of IFNγ, which is a potent macrophage-activating factor, inducing their tumoricidal activity. IFNγ-induced M1 macrophages are cancer-suppressive in vitro and in vivo, and produce pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNFα) as well as the Th1-polarizing cytokine IL-12. In a Th1 environment, pro-inflammatory cytokines play essential roles in cancer elimination by stimulating various aspects of the antitumor immune response. (Right) Cancer-promoting inflammation. In the absence of sufficient numbers of tumor-specific Th1 cells, tumor-infiltrating macrophages do not differentiate into a cancer-suppressive M1 phenotype. In this setting, pro-inflammatory cytokines may contribute to cancer development, progression and metastasis, for instance by stimulating angiogenesis and cancer cell growth and/or by increasing vascular permeability.