Figures & data
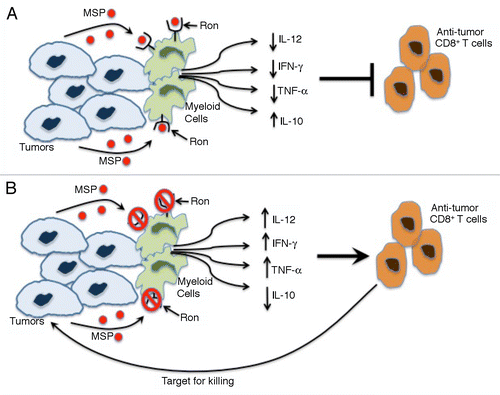
Figure 1. RON signaling suppresses antitumor CD8+ T-cell responses. (A) The binding of cancer cell-derived macrophage-stimulating protein (MSP) to RON stimulated myeloid cells to produce decreased levels of interleukin (IL)-12, interferon γ (IFNγ) and tumor necrosis factor α (TNFα) as well as increased amounts of IL-10. This cytokine profile suppresses antitumor CD8+ T cell responses and enables micrometastatic cancer cells to generate macrometastases. (B) The loss of RON signaling in the host, be it caused by genetic or pharmacological interventions, switches cytokine secretion by myeloid cells to a profile characterized by high levels of IL-12, IFNγ, and TNFα as well as by reduced amount of IL-10. This relieves immunosuppression, potentiating an antitumor CD8+ T-cell response that kills micrometastatic tumor cells.