Figures & data
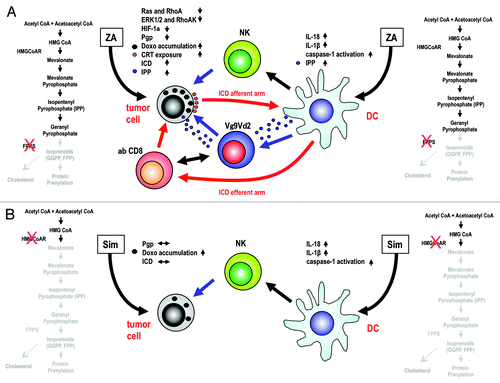
Figure 1. Consequences of mevalonate pathway inhibition in malignant cells and dendritic cells with zoledronic acid or simvastatin. (A) In tumor cells, the zoledronic acid (ZA)-mediated inhibition of farnesyl pyrophosphate (FPP) synthase (FPPS) results in decreased hypoxia-inducible factor 1α (HIF-1α) activity and limited P-glycoprotein (Pgp) expression, thus favoring the intracellular accumulation of doxorubicin (Doxo) and calreticulin (CRT) exposure. In this setting, dying cancer cells are engulfed by dendritic cells (DCs), representing the afferent arm of immunogenic cell death (ICD), and become able to prime antitumor cytotoxic T-cell responses, the efferent arm of ICD. ZA also induces the intracellular accumulation and release of isopentenyl pyrophosphate (IPP), leading to an increased functional activation of Vγ9Vδ2 T cells. In DCs, the ZA-dependent deprivation of isoprenoids stimulated the caspase-1-dependent production of interleukin (IL)-1β and IL-18, in turn promoting the activation of natural killer (NK) cells. ZA-treated DCs also release IPP, further activating Vγ9Vδ2 T cells, which are potent adjuvants for MHC-restricted αβ CD8+ T as well as NK cells. Thus, the afferent and efferent arms of ICD are boosted by the activation of Vγ9Vδ2 T cells. (B) The administration of simvastatin (Sim) to malignant cells inhibits cholesterol synthesis and hence limits the activity of the Pgp, thus favoring (to some extent) the intracellular accumulation of Doxo. However, Sim fails to decrease Pgp expression levels, to stimulate CRT exposure and to promote ICD. Moreover, the Sim-dependent inhibition of 3-hydroxy-3-methylglutaryl-CoA (HMGCoA) reductase (HMGCoAR) is not associate with the accumulation/release of IPP, implying that Sim is incapable of functionally recruiting Vγ9Vδ2 T cells. CoA, coenzyme A; GGPP, geranylgeranyl pyrophosphate.