Figures & data
Figure 1. ZnCl2 reactivates p53 in chemoresistant glioblastoma cells. (A) Semi-quantitative RT-PCR analyses of p53 target genes in human glioblastoma ADF cells pre-treated with 100 μM ZnCl2 for 6 h before the addition of 4 μg/ml cisplatin (cisp) for 16 h. When appropriate, the p53 inhibitor pifithryn α (PFT) (30 μM) was added to ZnCl2 before the administration of chemotherapy. β-actin levels were monitored as an internal standard. (B) 20000 ADF cells were plated in 60-mm dished and - 24 h later - treated with 100 μM ZnCl2 for 16 h, followed by the addition of 4 μg/mL cisp for 2 h. Cells were then washed with PBS and placed in fresh medium. ZnCl2 was replaced in the culture medium every two days. Colonies were stained with crystal violet 14 d after seeding and quantified. Data are presented as means ± SD *p = 0.034, as compared with cells treated with cispl plus ZnCl2.
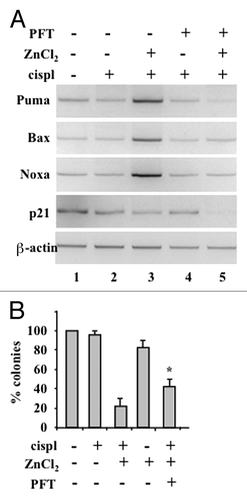
Figure 2. ZnCl2 exacerbates the cytotoxic potential of chemotherapy in chemoresistant cancer cells. Percentage of TUNEL+ human glioblastoma ADF cells, human glioblastoma U373 cells and colorectal carcinoma RKO-HIPK2i cells treated with 100 μM ZnCl2 for 6 h before the addition of 4 μg/mL cisplatin (cispl) (in U373 and ADF cells) or 2 μM adriamycin (ADR) (in RKO-HIPK2i cells) for 24 h. When appropriate, the pan-caspase inhibitor Z-VAD-fmk (40 μM) was added to ZnCl2 before the administration of chemotherapy. Data are reported as means ± SD *p = 0.001, as compared with untreated cells.
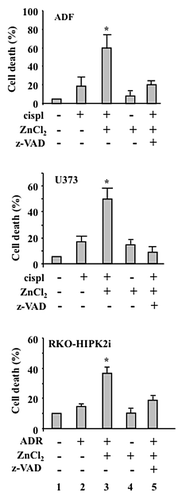
Figure 3. Chemoresistant cancer cells succumbing to chemotherapy in the presence of ZnCl2 promote dendritic cell (DC) maturation. (A) Human glioblastoma ADF cells, human glioblastoma U373 cells and colorectal carcinoma RKO-HIPK2i cells were treated with 100 μM ZnCl2 for 6 h, followed by the addition of 4 μg/mL cisplatin (cispl) (in U373 and ADF cells) or 2 μM adriamycin (ADR) (in RKO-HIPK2i cells) for 16 h. Thereafter, tumor cells were co-cultured with immature dendritic cells (DCs) for 24 h, followed by the cytofluorometric assessment of the DC maturation markers CD83 and CD86. Data from one representative experiment are reported as the mean percentage of CD83+ and CD86+ DCs ± SD *p = 0.001, as compared with untreated cells. (B) ADF cells were treated with 100 μM ZnCl2 alone or in combination with 40 μM Z-VAD-fmk for 6 h, followed by the administration of 4 μg/mL cisp for 16 h. Thereafter, cells were co-cultured with DCs and DC maturation markers assessed as in Panel A. Results from one representative experiment are shown as means ± SD (C) Upper panel: percentage of TUNEL+ wild-type (wt) RKO cells treated with 2 μM ADR for 24 h. When appropriate, 40 μM Z-VAD-fmk was added for 1 h before ADR administration. Data are reported as means ± SD. Lower panel: immunoblotting assessment of PARP cleavage in RKO cells treated with ADR alone or in combination with Z-VAD-fmk. l.c., loading control. (D) RKO cells treated as in Panel C were co-cultured with immature DCs for 24 h, followed by the cytofluorometric assessment of CD86. Data for one representative experiment are reported as the percentage of CD86+ DCs ± SD (E) Total and membrane proteins were extracted from equal amounts of RKO cells exposed to 2 μM ADR for 24 h and immunoblotted with a calreticulin (CRT)-specific antibody.
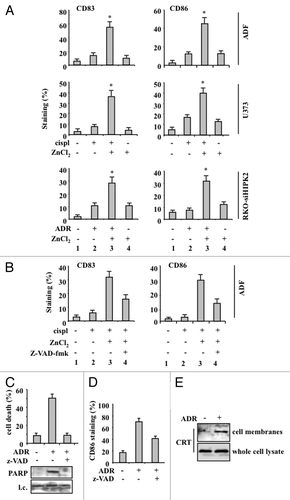
Figure 4. ZnCl2 induces calreticulin exposure on the membrane of chemoresistant cancer cells. (A) Human glioblastoma ADF cells and colorectal carcinoma RKO-HIPK2i cells were treated with 100 μM ZnCl2, 4 μg/mL cisplatin (cispl) or 2 μM adriamycin (ADR), as indicated, for 16 h. Thereafter, total and membrane proteins were extracted from equal amounts of RKO cells and immunoblotted with a calreticulin (CRT)-specific antibody. (B) CRT exposure by living human glioblastoma U373 cells exposed to 100 μM ZnCl2 and 4 μg/mL cisp, alone or in combination, was verified by flow cytometry. Data, which are representative of two independent experiments, are reported as means ± SD (C) ADF, U373 and RKO-HIPK2i cells were treated with 100 μM ZnCl2 alone or in the presence of 10 μM brefeldin A (Bref A), for 16 h. Membrane proteins extracted from equal amounts of cells were assayed by immunoblotting with an anti-CRT antibody. (D) ADF cells were treated with 100 μM ZnCl2 for the indicated time (h), followed by the extraction of total proteins from equal amounts of cells and immunoblotting with antibodies specific for the indicated proteins. β actin levels were monitored as a loading control.
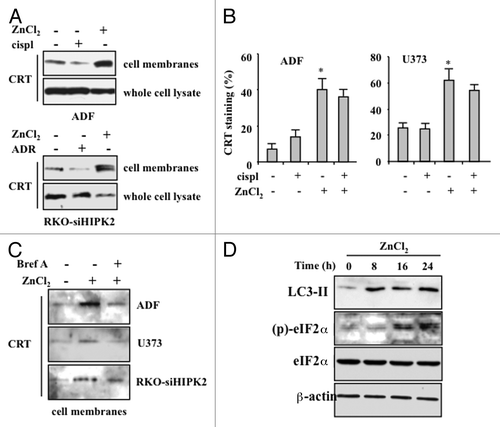
Figure 5. The inhibition of calreticulin exposure by brefeldin A impairs dendritic cell activation by chemoresistant cancer cells succumbing to chemotherapy in the presence of ZnCl2. (A and B) Human glioblastoma ADF cells were treated with 100 μM ZnCl2 alone or together with 10 μM brefeldin A (BrefA) for 6 h, followed by the administration of 4 μg/mL cisplatin (cispl) for additional 16 h. Thereafter, cancer cells were co-cultured with immature dendritic cells (DCs) for 24 h and the DC maturation markers CD83 and CD86 were monitored by flow cytometry. Data from one representative experiment are reported as staining profiles (A), isotype controls in black) or mean percentage of CD83+ and CD86+ DCs ± SD; *p = 0.001 (B).
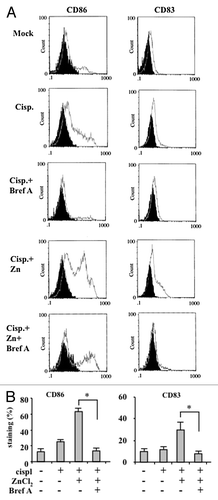
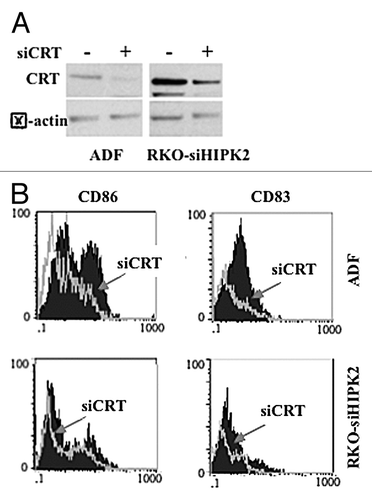
Figure 6. Calreticulin depletion in cancer cells impairs their capacity to activate dendritic cells in the course of immunogenic cell death. (A) Human glioblastoma ADF cells and colorectal carcinoma RKO-HIPK2i cells were transfected with a control siRNA (-) or a siRNA specific for calreticulin (siCRT; +). 36 h after transfection, cells were assayed by immunoblotting for calreticulin (CRT) expression levels. β actin was monitored as a loading control. (B) Control ADF and RKO-HIPK2i cells (empty gray histograms) or ADF and RKO-HIPK2i subjected to CRT depletion as in Panel A (black-filled hystograms) were treated with 100 μM ZnCl2 for 6 h, followed by the administration of 4 μg/mL cisplatin (cispl; in ADF cells) or 2 μM adriamycin (ADR; in RKO-HIPK2i cells) for 16 h cancer cells were co-cultured with immature dendritic cells (DCs) for 24 h and the DC maturation markers CD83 and CD86 were monitored by flow cytometry. Data from one representative experiment are reported as staining profiles.