Figures & data
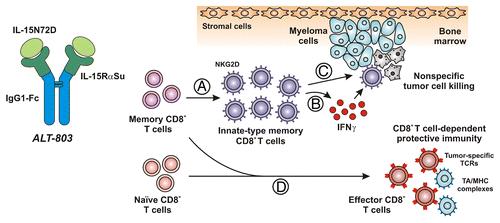
Figure 1. ALT-803 promotes innate-like CD8+ T-cell effector activity and protective antitumor immunity in myeloma-bearing mice. ALT-803 is a supramolecular complex that exhibits superagonist activity and is comprised of a mutant form of interleukin-15 (IL-15N72D) associated with a dimeric IL-15 receptor α chain sushi domain (IL-15RαSu)–IgG1 Fc fusion. The N72D substitution confers to IL-15 increased affinity for the IL-2 receptor β chain (IL-2Rβ) and enhanced biological activity. In addition, association of IL-15N72D with IL-15RαSu further improves the biological activity of IL-15 in vivo, resulting in the potent activation of IL-2Rβ/γ-bearing natural killer (NK) cells and T lymphocytes. (A) In myeloma-bearing mice, ALT-803 promoted the rapid expansion of memory CD8+ T cells but not naïve CD8+ T lymphocytes. (B) Such memory CD8+ T cells secreted high levels of interferon γ (IFNγ) and upregulated killer cell lectin-like receptor subfamily K, member 1 (KLRK1, best known as NKG2D) but not of programmed cell death 1 (PDCD1, best known as PD-1) and CD25, on their surfaces. (C) ALT-803-activated cells also mediated nonspecific cytotoxicity against myeloma cells and other tumor cells, via a mechanism that was partially dependent on IFNγ. By activating such a response, ALT-803 was capable of eliminating well-established myelomas from the bone marrow and significantly prolonging the survival of tumor-bearing mice. (D) Short-term ALT-803 treatment also provided tumor-bearing mice with protective immunity against a subsequent inoculation of myeloma cells. This protective response appeared to rely on CD8+ T lymphocytes. Presumably, ALT-803 treatment stimulated naïve and/or memory CD8+ T cells specific for tumor-associated antigens (TAAs) to acquire effector functions against a subsequent tumor challenge.