Figures & data
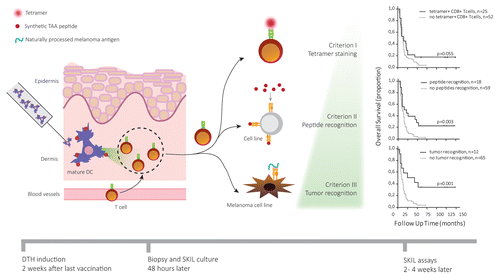
Figure 1. Analysis of skin-infiltrating lymphocytes allows for early prediction of clinical responses in cancer patients treated with immunotherapy. Dendritic cells (DCs) generated, activated, and loaded with tumor-associated antigens (TAAs) ex vivo were injected in the dermis of melanoma patients 1 to 2 wk after vaccination. The resulting delayed type hypersensitivity (DTH) reaction promotes the migration of TAA-specific T cells into the skin, which were isolated via punch biopsy 48 h after injection. Isolated skin-infiltrating lymphocytes (SKILs) were expanded and tested for cancer-specificity using tetramer staining (criterion 1). Patients whose SKIL cultures contained antigen-specific CD8+ T cells showed a survival benefit over patients not fulfilling this criterion. Additionally, the specific recognition of TAA-derived peptides by SKILs (criterion II) was measured in terms of cytotoxic activity or secretion of TH1 cytokines, such as interleukin (IL)-2, tumor necrosis factor α (TNFα) and interferon γ (IFNγ), coupled to the release of no TH2 cytokines (i.e., IL-4, IL-5, IL-13). Finally, the ability of SKILs to specifically recognize TAAs naturally processed and presented by a melanoma cell line (criterion III) was assessed. Combining criterion 2 and 3 to criterion 1 further increased the predictive value of our assay.