Figures & data
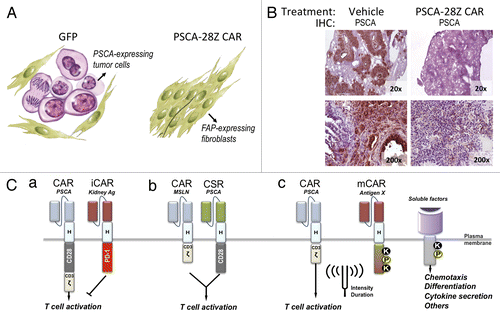
Figure 1. Treatment with anti-PSCA CAR and potential safety strategies to prevent on target/off tumor toxicity. (A) Schematic representation of the results previously obtained in a humanized mouse model of pancreatic cancer. Nod/SCID gamma (NSG) mice bearing human pancreatic adenocarcinoma (HPAC) subcutaneous xenografts were treated with human CD8+ T cells transduced with GFP (control) or with an anti-prostate stem cell antigen (PSCA) chimeric antigen receptor (CAR). Forty days after treatment, residual tumors were excised and analyzed for PSCA expression. (B) Immunohistochemical staining of PSCA in lung sections from mice at the end of the treatment. Magnification is indicated at the bottom right corner of each micrograph. (C) Potential strategies to restrict cytotoxicity of CAR-transduced cells to tumor tissue. (a) Coexpression of anti-PSCA CAR, together with an inhibitory CAR (iCAR) directed to an antigen expressed in critical normal tissues that express PSCA. (b) The T cell activation (CD3-zeta) and costimulatory (CD28) domains can be split into two different receptors, each targeting a different antigen, such that ligation of both receptors is necessary to unleash the cytotoxic potential of T cells. CSR: Costimulatory receptor. (c) Combination of CARs with more sophisticated modulatory receptors (mCAR) to ‘fine tune’ the biological activity of CAR-transduced lymphocytes in terms of intensity and/or duration. Synthetic signaling domains can be generated that contain docking sites for kinases (K), phosphates (P) or other signal transducers allowing for the activation of signaling cascades in response to virtually any antigen.