Figures & data
Figure 1. OCRL1 (c-terminal portion) in complex with Rab8a and the F&H peptide of Ses1. Rab proteins and F&H motif proteins bind to two distinct binding sites. Rab8a is binding to the ASH domain and to a c-terminal α-helix of the inositol-5-phosphatase domain. Ses1 binds to the posterior surface of the RhoGAP-like domain.
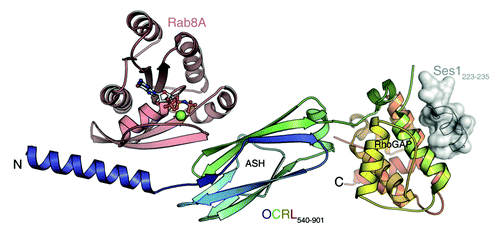
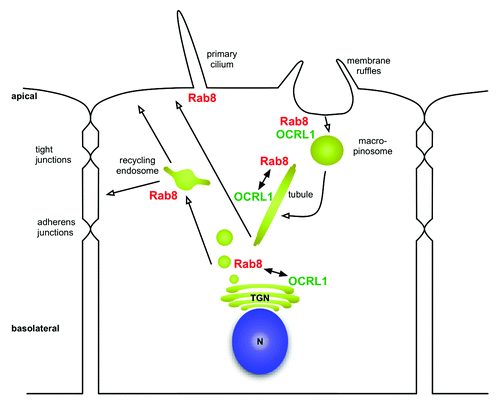
Figure 2. Overview of subcellular distribution of Rab8 and OCRL1 . Rab8 is localized to membrane ruffles and regulates transformation of macropinosomes into tubules. Rab8 also regulates transport to the recycling endosome within the biosynthetic pathway and is involved in the biogenesis of cilia. Potential involvement of OCRL1 is discussed in the main text. n = nucleus; TGN = Trans-Golgi-Network.