Figures & data
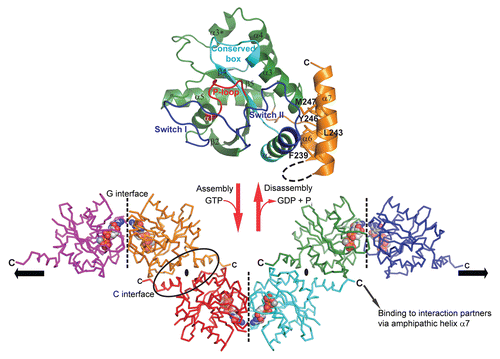
Figure 1 GTP-dependent scaffold formation of GIMAP2. (upper part) The structure of the monomeric nucleotide-free GIMAP2 shows a Ras-like G-domain (in green). Additional secondary structure elements compared to the minimal Ras G-domain are helix α3* and the two amphipathic helices α6 and α7 (in orange), which fold against switch II (blue). (lower part) In the absence of α7 and the presence of GTP, GIMAP2 oligomerized via two interfaces in the crystal to form a linear oligomer (the direction of the oligomerization axis is indicated by the black arrows). The conversion between the monomeric and oligomeric states is regulated by GTP binding or hydrolysis (red arrows).