Figures & data
Figure 1 (A) Schematic showing the recognized cellular functions of FAK, highlighting the direction-sensing and polarization functions we describe here. (B) Images show comparison of cell shape and actin filaments in FAK-deficient SCC cells re-expressing either FAK-wt or the FAK/RACK1 binding impaired mutant FAK-E139A,D140A. (C) The co-staining of RACK1 and PDE4D5 is shown in protrusive nascent adhesions as FAK-wt SCC cells are plated on to FN for 15 min (solid arrows). By comparison, both RACK1 and PDE4D5 are cytoplasmic in FAK-E139A,D140A-expressing cells (broken arrows). Scale bars 20 µm.
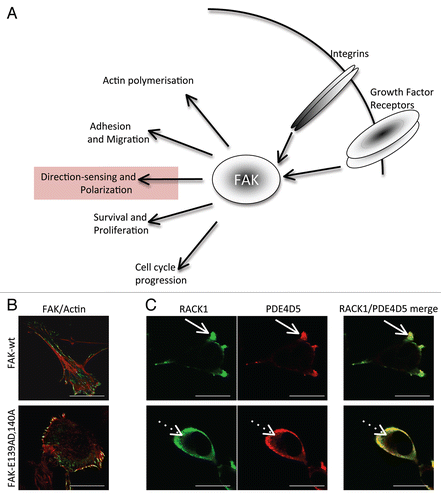
Figure 2 (A) FAK-wt or FAK-E139A,D140A cells (as indicated) were plated on FN for 2 hours and wounded in the presence of 8-CPT-2OMe cAMP (CPT-cAMP; 10 µM), KT 5720 (1 µM), 8-Brm-cAMP (10 µM), KT 5720 + 8-Brm-cAMP or N6-Bnz-cAMP (10 µM). After 1.5 hours cells were fixed and stained with the Golgi marker anti-GM130, TRITC phalloidin and DAPI, to assess polarization in response to the wounded areas. The percentage of each cell type with the Golgi orientated to the wound was calculated by counting 100 cells in 3 experiments and is shown in the graph. (B) FAK-wt cells were treated with KT 5720 (1 µM) and/or N8 = Brm-cAMP (left parts) and with 6-Bnz-cAMP (10 µM) for 30 min then immunoblotted using anti-phospho-CREB and actin antibodies as probes. (C) FAK-wt cells with or without CPT-cAMP (10 µM for 3.5 hrs, time point chosen to maintain consistency with cell polarization experiments, conditions detailed above; left part) or FAK-wt and FAK-E139A,D140A cells (right part), were harvested and a Rap1 assay performed as described in Materials and Methods. Quantification of Rap1-GTP normalized to total Rap1 from three separate experiments is shown.
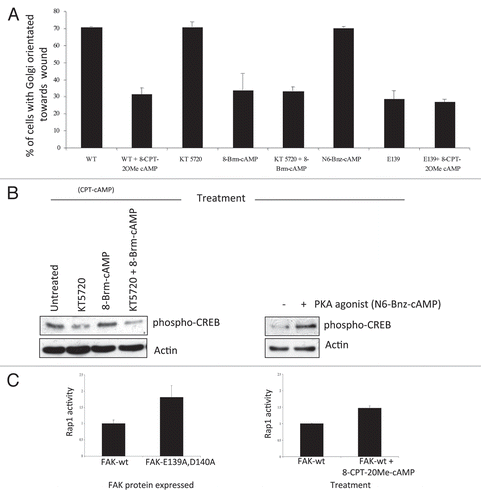
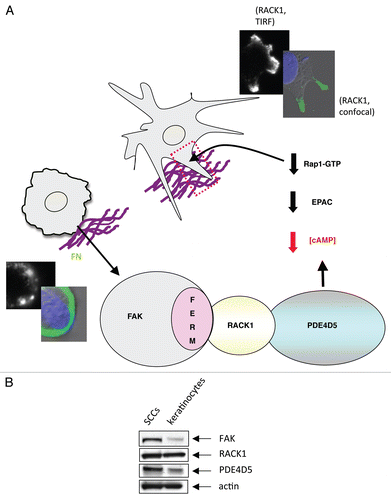
Figure 3 (A) Model outlining how our data explains the role of the FAK/RACK1/PDE4D5 complex in keeping cAMP levels low in the vicinity of nascent peripheral adhesion structures as these form and sense their environment. In our model, this is required for a sub-set of nascent adhesions to stabilize, typically along one cell edge, so inducing a polarized phenotype. The FAK/RACK1-mediated recruitment of PDE4D5 signals to Rap1, likely via EPAC, to keep Rap1 appropriately suppressed in space and time so as to permit nascent adhesion stabilization, spreading and polaization. This is in keeping with our earlier data recently published in reference Citation4. The edges of cells expressing FAK-wt (top images showing stabilized protrusive structures) and FAK-E139A,D140A (lower images) are shown, including TIR F images (black and white) taken at the cell-substratum interface. (B) Expression levels of FAK, RACK1 and PDE4D5 were determined in lysates from normal and malignant keratinocytes (SCCs) which were immunoblotted using anti-FAK, anti-RACK1, anti-PD4D5 and anti-actin antibodies as probes.