Figures & data
Figure 1. Overview of Rac1-interacting proteins. In the left part of the figure, proteins known to bind to the effector domain (ED) of Rac1 via their CRIB domain or not are listed. In the right part of the figure, proteins known to bind to the hypervariable (HV) region are listed. These proteins are clustered dependent on their known interactions. See text for details.
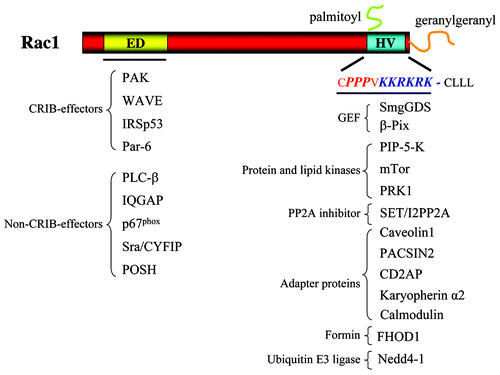
Figure 2. Rac1 interactions at Focal Adhesions and in the nucleus. Figure provides a model of the different local interactions of Rac1 with the various C-terminal-interacting proteins. The various regions that focus Rac1 activity and regulation are indicated by the different colors. Integrin-mediated Rac1 activation, possibly by PIX, leads to its targeting to FAs and recruitment of Caveolin1. Caveolin1 subsequently regulates Rac1 ubiquitylation and internalization. Internalized Rac1 may associate to Karyopherin α, translocate to the nucleus for further ubiquitylation and proteasomal degradation.
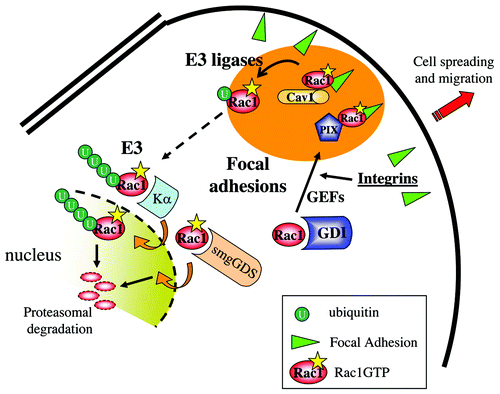
Figure 3. Internalization of activated Rac1 and binding to effector kinases. Activated Rac1 may interact with a series of protein kinases at the plasma membrane. These represent primarily effector kinases such as PAK, PRK, PIP-5-K and mTor. Active Rac1 at the membrane also associates with PACSIN2, which regulates Rac1 internalization toward early endosomes, paralleled by GAP-mediated Rac1 inactivation.
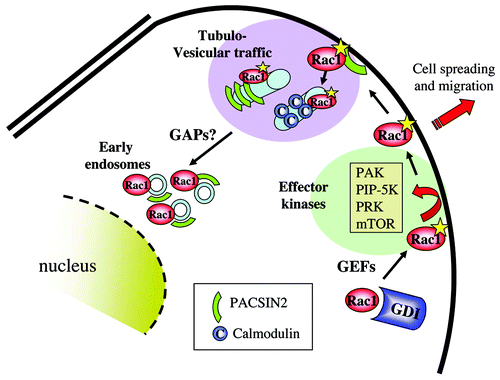
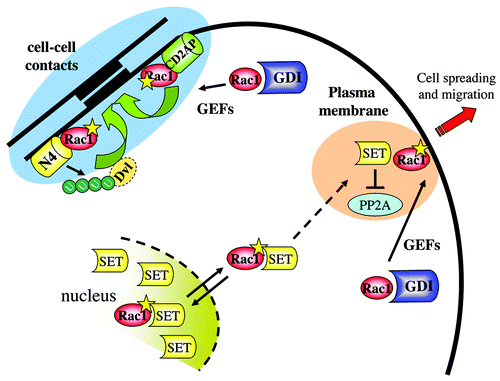
Figure 4. The nuclear connection for Rac1 and its role in cell–cell contacts. Activated Rac1 recruits the nuclear protein SET and/or a Rac1-SET complex from the nucleus to the plasma membrane to regulate cell migration. Conversely, at cell–cell contacts, activated Rac1 recruits CD2AP as well as Nedd4 (N4) to promote intercellular adhesion. The latter pathways also involves Nedd4-mediated ubiquitylation of the adaptor protein disheveled (Dvl1).