Figures & data
Figure 1. Kinase-dependent and -independent roles of Cdc28. (A) In late G1, Cdc28 phosphorylates the SBF-bound repressor Whi5. This triggers the release of Whi5 from the SBF, thus stimulating the transcription of the genes belonging to the G1 transcriptional program. (B) Cdc28/Cks1 and the 19S-regulatory particle of the proteasome are recruited to genes such as GAL1 for efficient transcriptional activation by promoting a decrease in nucleosomal density.
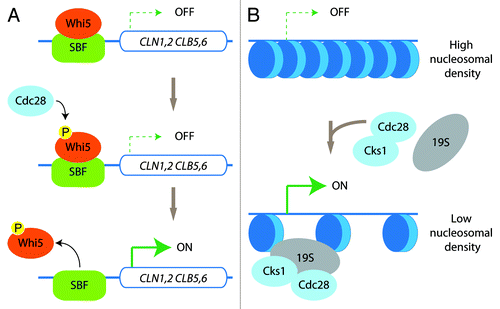
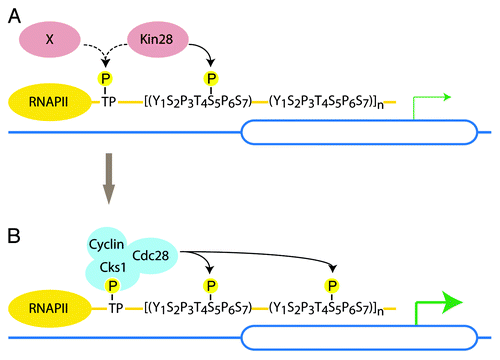
Figure 2. Model for Cdc28-mediated phosphorylation of the CTD. (A) In late G1 Kin28 (or potentially a still uncharacterized kinase) primes the CTD. (B) Phosphorylated TP sites (threonine followed by a proline) and/or multiple phospho-S5 serve as a docking site for Cks1/Cdc28. As Cdc28 becomes active in late G1, Cdc28 further phosphorylates S5 to ensure high rates of transcription and mRNA capping.