Figures & data
Figure 1 Toll-deficient Drosophila flies. Image of a (A) male and a (B) female D. melanogaster. The arrows point to their genitalia. (C) A female virgin D. melanogaster. The arrow points to the embryonic residue that is present in virgin female flies within the first 8–12 h after eclosion. (D and E) The “multiple-hair type” of bristle seen in flies with the TM6B balancer, such as TlI-RXA/TM6B and Tlr632/TM6B flies (E is an image of the bristle in D at a higher magnification). (F and G) The “double-hair type” of bristle seen in flies without the TM6B balancer, such as Tlr632/TlI-RXA flies (G is an image of the bristle in F at a higher magnification).
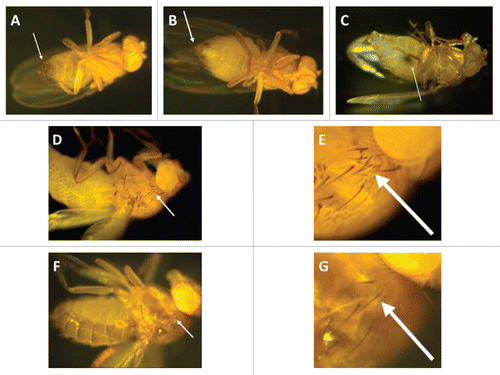
Figure 2 Infection assays of aspergillosis in Toll-deficient D. melanogaster. (A) Anesthetized flies on a CO2-flow fly pad. (B) Injection assay. A CO2-anesthetized fly was pricked at its dorsolateral thorax with a 0.1 mm diameter needle previously dipped in a concentrated Aspergillus conidial solution. (C) Rolling assay. Anesthetized flies were rolled on a Petri dish covered by a fresh layer of Aspergillus conidia for 2 min. At the end of rolling, Aspergillus uniformly covered the fly surface. (D) Fifteen milliliters of a sterile YAG medium that was allowed to solidify in an empty vial. (E) Ingestion assay. Flies feeding on the surface of a fresh lawn of Aspergillus conidia pre-grown in a YAG-containing vial.
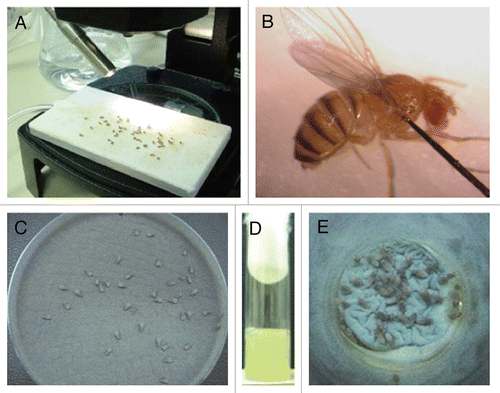
Figure 3 Preparation of antifungal-drug containing food vials and fly fasting. (A) A spatula is used to create 2- to 3-mm-deep abrasions on the surface of the fly food. Afterward, 200 µl of the antifungal drug of choice is added to the surface. (B) Dry yeast particles are then added to the surface of the drug-containing vial until they are entirely soaked by the antifungal agent. (C) Before exposure to the drug-containing food vials, flies are placed in empty vials for 6–8 hours so that they can starve.
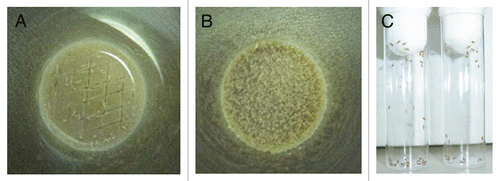
Figure 4 Toll-deficient Drosophila flies are susceptible to Aspergillus challenge. Shown are Kaplan-Meier survival curves for OregonR WT and Tlr632/TlI-RXA flies infected with AF293 using the (A) injection, (B) rolling and (C) ingestion assays. (D) Aspergillus conidia covering a fly's exterior surface (arrow) following the rolling assay. (E) Aspergillus conidia in the lumen of a fly's gastrointestinal tract (black arrow) following the ingestion assay. Later on, conidia invade through the gastrointestinal tract (white arrow) and cause disseminated infection.
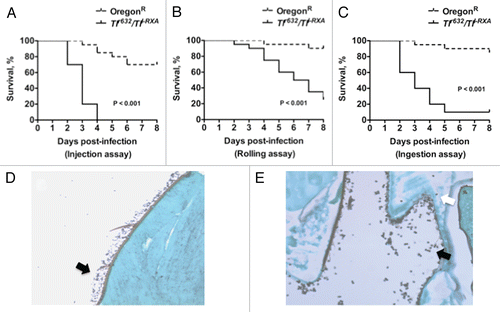
Figure 5 Evaluation of Aspergillus virulence in Toll-deficient Drosophila flies. (A) Survival rates in Tlr632/TlI-RXA flies following injection of the hypovirulent ΔgliP Aspergillus strain or its isogenic WT strain AF293. (B) Survival rates in Tlr632/TlI-RXA flies eight days after infection with the hypovirulent Δalb1 Aspergillus strain compared with infection with its isogenic WT strain B-5233 in the three infection assays. (C) qPCR analysis of the tissue fungal burden in Tlr632/TlI-RXA flies infected with Δalb1 Aspergillus or its isogenic WT strain in the rolling assay. CE, conidial equivalent of Aspergillus fumigatus DNA.
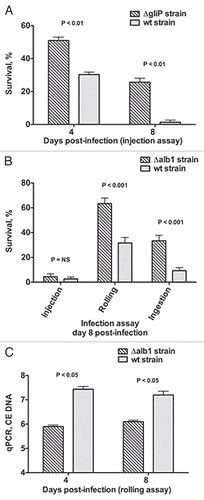
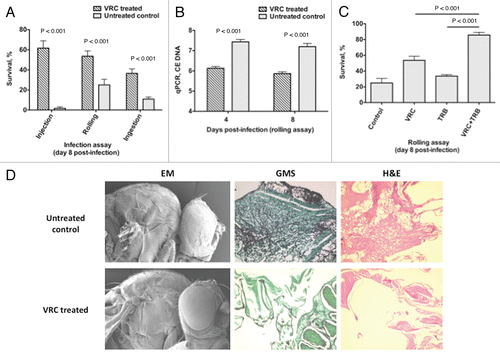
Figure 6 Voriconazole protects Toll-deficient Drosophila flies against Aspergillus infection. (A) Survival rates in untreated control and voriconazole-treated Tlr632/TlI-RXA flies 8 days after infection with AF293 in the three infection assays. (B) qPCR analysis of the tissue fungal burden in untreated control and voriconazole-treated Tlr632/TlI-RXA flies infected in the rolling assay. (C) Survival rates in untreated control and Tlr632/TlI-RXA flies given voriconazole, terbinafine or a combination of the two drugs 8 days after infection with AF293 in the rolling assay. (D) Histopathological and scanning electron microscopic analysis of the difference in tissue fungal burden in untreated control and voriconazole-treated Tlr632/TlI-RXA flies. VRC, voriconazole; TRB, terbinafine; EM, electron microscopy; GMS, Grocott-Gomori methenamine-silver nitrate stain; H&E, hematoxylin-eosin stain; CE, conidial equivalent of Aspergillus fumigatus DNA.