Figures & data
Figure 1 Description of the reporter enzyme fluorescence (REF) approach to imaging bacterial infections. (A) The reaction that generates RE F signal is catalyzed by a bacterial enzyme, preferably naturally produced. In this case, the bacteria is Mycobacterium tuberculosis (Mtb) and the enzyme is β-lactamase. A custom substrate is used that either is based on fluorescence resonance energy transfer (FRET) quenching of the fluorophore or uses a structure that is not fluorescent until cleavage. (B) The structure of CNIR5, one of the primary substrates used successfully for REF. The β-lactam ring indicated is cleaved by β-lactamase and separates the fluorescent dye, in this case Cy5.5, from the quencher (QSY22) that absorbs light at the Cy5.5 wavelength of emission (∼690 nm). Modifications that impact catalytic activity and specificity can include changes to any component of this structure, but those substitutions surrounding the β-lactam ring have the greatest impact. (C) Proposed model that outlines the high sensitivity of REF. The substrate (CNIR5) is cleaved to a product by intracellular Mtb. The product (Cy5.5) is retained within the host cell and builds up to very high levels until substrate is no longer available.
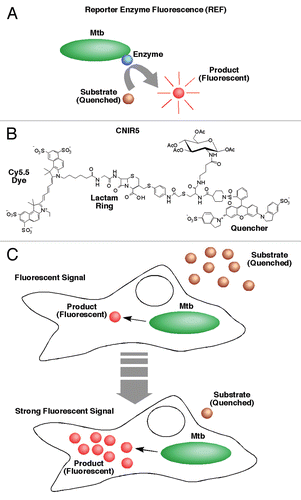
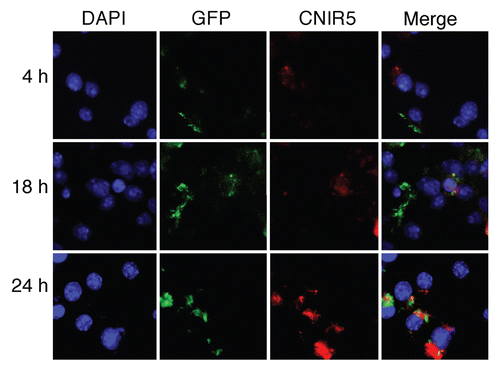
Figure 2 Observed fluorescent product from REF accumulating in host macrophages over time post-infection with mycobacteria. Host cell (J774A.1 murine macrophages) nuclei were stained with DAPI (blue), mycobacteria were expressing GFP (green) and the CNIR5 fluorescent dye is Cy5.5 (red). Note the increase in product (red) fluorescence between four and 24 h post-infection with mycobacteria. This confocal microscopy supports the model in . A pool of fluorescent product is visible within infected cells that is sufficient for fluorescence-activated cell sorting (FACS) and confocal microscopy, explaining the high levels of signal observed over time, even when animals are infected with low bacterial numbers.