Figures & data
Figure 1. Crossing of the blood-brain barrier (BBB) by parasites associated with WBCs. (A) Illustration of a cerebral post-capillary vessel showing the BBB, consisting of a complex of cerebral endothelial cells and their tight junctions, basement membranes and pericytes as well as astrocytic end-feet. Note the perivascular space that is noticeable during inflammation. (B) Toxoplasma gondii crossing the BBB. (1) Induction of vascular cell adhesion molecule 1 (VCAM-1) and adhesion molecules activated leukocyte cell adhesion molecule (ALCAM) in the brain during infection might aid the monocytes infected Toxoplasma gondii tachyzoites to cross the BBB. The infection cause an increased motility of the monocytes which may facilitate their migration into the brain. Toxoplasma gondii can invade endothelial cells, however, its ability to cross the BBB as extracellular parasites in vivo is not clear. (C) The extracellular parasite Trypanosoma brucei spp and T cells cross the endothelial cell layer, the endothelial basement membrane and the parenchymal to invade the brain parenchyma. (1) TNFα and IFNα/β are released upon TLR9/MyD88-mediated activation of the innate immune response. (2) TNFα induces ICAM/VCAM on cerebral endothelial cells which allow attachment of T cells. IFNα/β induces a limited release of CXCL10 by endothelial cells and/or astrocytes, which is enough for penetration of T cells accompanied by some trypanosomes into the perivascular space. (3) Trypanosome-derived antigens taken up and expressed by macrophages could then be recognized by sensitized T cells to induce IFNγ, which augment the process through (4) induction of CXCL10 production by astrocytes and (5) molecules that open the parenchymal basement membrane for spread of both T cells and trypanosomes into the brain parenchyma. (6) Once inside the brain the parasites are most likely controlled by macrophages or microglia which produce trypanotoxic or static molecules.
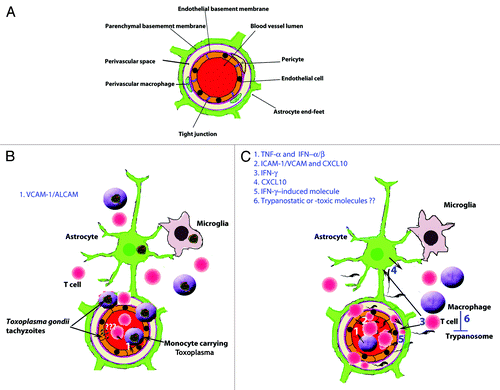
Figure 2. Large accumulation of T. b. brucei (red) in the choroid plexus following intra-peritoneal injection in a rodent model. Choroid plexus loaded with trypanosomes is seen already one week after the infection and before trypanosome crossing of the BBB, of which timing and prevalence is dependent on the rodent strain.