Figures & data
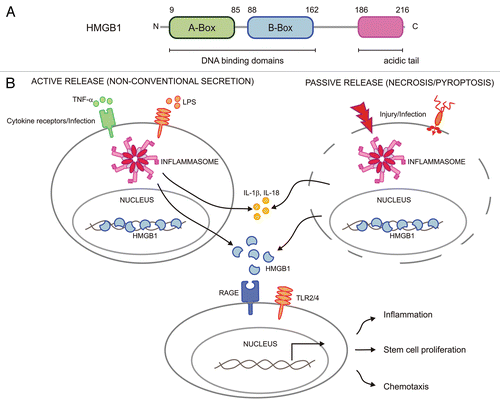
Figure 1 Structure of HMGB1 and its functions in the extracellular environment. (A) Human HMGB1 is a protein of 216 amino acids that is composed of two homologous DN A-binding domains called ‘A and B boxes’ that are followed by a negatively charged acidic tail in the carboxyl-terminus. (B) HMGB1 can be secreted through non-canonical mechanisms along with pro-inflammatory cytokines such as IL-1β and IL-18 following inflammasome activation in stimulated and/or infected immune cells, or it may be passively released from damaged and infected cells undergoing necrotic or pyroptotic cell death. Extracellular HMGB1 can bind to its receptors RAGE and TLR2/4 on effector cells in order to induce inflammation, chemotaxis and repair responses.