Figures & data
Figure 1. HCMV-infected monocytes fail to differentiate into MoDCs upon cytokine stimulation. HCMV infection of moDCs further impairs the function of these cells, whose maturation is impaired both by HCMV infection and the virally encoded protein cmvIL-10. HCMV infection of moDCs impairs the ability of the DCs to present antigens to T cells and subsequent T-cell proliferation, through mechanisms such as downregulating class II and CD1 expression, inducing apoptosis of T cells via CD95L and TRAIL, and altering cytokine production as well as mechanisms involving release of soluble CD83 and virally encoded UL18 and cmvIL-10. HCMV infection of moDCs also impairs the ability of the DCs to migrate in response to chemokines by causing the cell to internalize CCR1 and CCR5, by inhibiting the switch from CCR5 to CCR7, and by mechanisms involving virally encoded UL18.
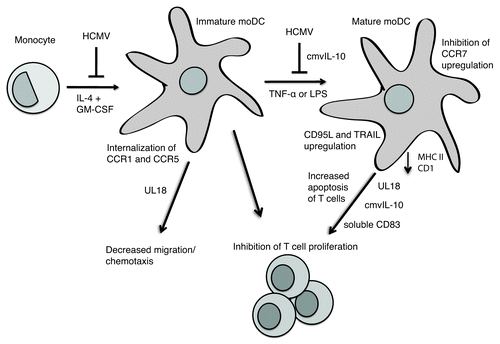
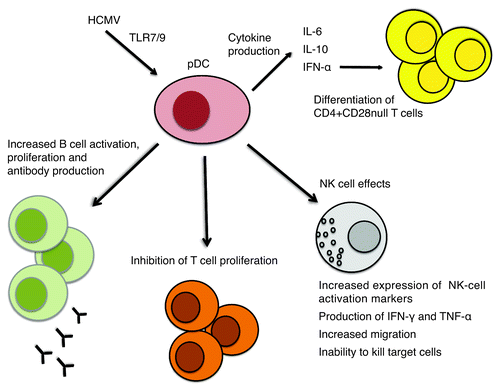
Figure 2. HCMV infects pDCs nonpermissively. Through engagement of the TLR7 and/or TLR9 pathways, HCMV induces production of IFN-α and secretion of IL-6 and IL-10. HCMV infection in pDCs has an inhibitory effect on T-cell proliferation, but triggers B-cell activation and proliferation and antibody production. HCMV infection of pDCs has a pronounced effect on NK cells: it increases expression of NK-cell activation markers, induces production of high levels of inflammatory cytokines, and increases migration, but impairs the ability to kill target cells. IFN-α production by HCMV-infected pDCs together with chronic HCMV antigen stimulation may lead to differentiation of CD4+CD28-null T cells.