Figures & data
Figure 1. Schematic of PVL pore formation, and the different cellular pathways it uses to activate host cells. Steps involved in immune activation are outlined in solid boxes, while steps in pore formation are outlined in gray dashed boxes. Sublytic levels of PVL activate human neutrophils by stimulating calcium ion channels, followed by an influx of calcium into the cell. This occurs prior to PVL pore formation. PVL has also been shown to activate murine macrophages via TLR2. While sublytic PVL do not require TLR to prime human neutrophils, it has been suggested that PVL lysis of host cells releases host damage-associated molecular patterns (DAMPs) that in turn is recognized by TLR to activate human neutrophils and macrophages/monocytes.
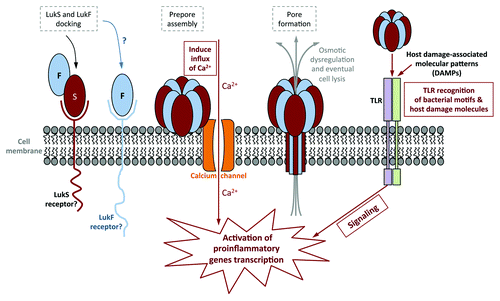
Figure 2. Comparison of the virulence of PVL-producing MRSA strains with their respective isogenic ∆pvl mutants in a mouse pneumonia model. (A) Comparison of the percent mortality at 48 h in mice infected with three different MRSA strains, their ∆pvl isogenic strains and, in the case of strain MW2, the PVL-complemented strain (pvl comp). p values were determined by Chi-square analysis. Differences with strain NRS193 were not significant. (B) Survival curves comparing outcomes in mice infected with WT PVL+ MRSA or isogenic strains expressing higher levels of PVL from pOS1-pvl. p values by log-rank test. (C) Trend correlating levels of PVL production and survival in a mouse model of S. aureus pneumonia. (D) Pathology of selected murine lung sections 8 h post infection with MRSA strain MW2, and its isogenic ∆pvl mutant.
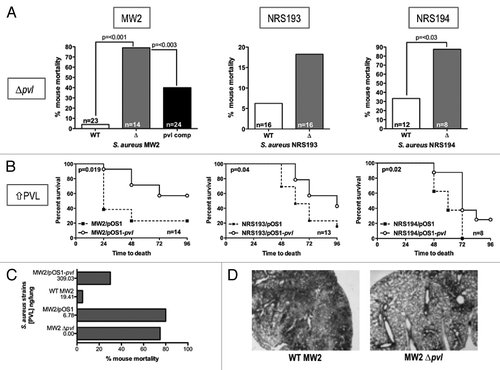
Figure 3. Immunomodulatory effects of PVL on mouse cells. (A and B) Detection of phospho-p38 and murine KC produced by purified PMNs from FVB mice (A), and mouse lung epithelial cells MLE12 (B) exposed to indicated concentrations of purified PVL. (C) Percentages of viable MRSA strain MW2 after addition of supernatants from the indicated cells that were first incubated with purified PVL, compared with bacterial count in cells lacking exposure to PVL. Bacterial counts from cell supernatant killing assays are averaged from a minimum of 3 independent experiments. Error bars denote SEM. Statistical analyses were performed by the t-test (***p < 0.01; **p < 0.05; *p < 0.01). (D) Detection of TNFα in murine pulmonary tissues infected with WT or Δpvl MRSA strain MW2 18 h after intranasal infection with 5 × 108 cfu/mouse. (E) Production of Caspase 3, as determined by immunoblot, from indicated cells 6 h after exposure to indicated concentration of PVL. ◆ denotes PVL concentrations that stimulate release of antibacterial factors by cells.
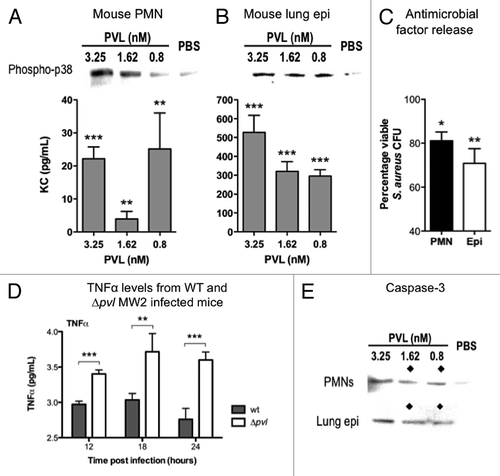
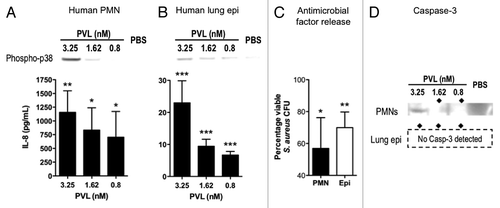
Figure 4. Immunomodulatory effects of PVL on human cells. (A and B) Detection of phospho-p38 and human IL-8 produced by purified PMNs (A) and cultured A549 human alveolar basal epithelial cell line (B) exposed to indicated concentrations of purified PVL. (C) Percentages of viable MRSA strain MW2 after addition of supernatants from the indicated cells that were first incubated with purified PVL, compared with bacterial count in cells lacking exposure to PVL. Bacterial counts from cell supernatant killing assays are averaged from a minimum of 3 independent experiments. Error bars denote SEM. Statistical analyses were performed by the t-test (***p < 0.01; **p < 0.05; *p < 0.01). (D) Production of Caspase 3, as determined by immunoblot, from indicated cells 6 h after exposure to indicated concentration of PVL. ◆ denotes PVL concentrations that stimulate release of antibacterial factors by cells.