Figures & data
Figure 1. Hamster sCJD progression in days post-infection ic. Superimposed data for infectious titer in logs (red solid line) and % PrP-res of the total PrP (blue broken line). PrP is not converted to PrP-res until 80 d, and PrP-res shows a late onset and much steeper trajectory than infectivity.Citation34 The effective doubling time of PrP-res is 65× the rate of agent doubling; this rate includes both replication and destruction (see text). Note clinical signs appear at beginning of the high plateau phase of infectivity and PrP-res.
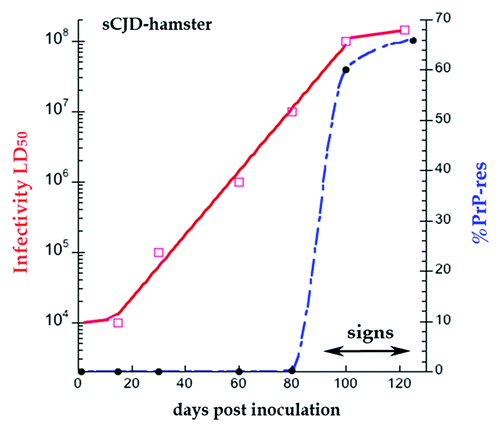
Figure 2. Late onset of PrP-res in FU-CJD wt mice illustrates constant level of PrP in left panel without proteinase K (PK) from 50–130 d. Right panel shows the FU-CJD agent replicates by 5 logs in these mice before PrP-res is first detectable at 90 d (+PK lanes) as shown by the interpolated rise between assay points at ~15 and 100 d. Gel load at 130 d was decreased to 0.5× for detection in the linear range. Log infectivity increase by ic route has been reproduced (n > 3 from different serial passages).
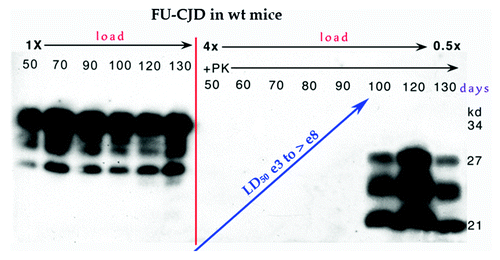
Figure 3. Model of invading ~25 nm TSE viral particle binding its required host PrP receptor to induce PrP-res amyloid. (A) Shows disintegration and elimination of the infectious particle with its nucleic acid core (solid circle) and protective protein capsid cage when there is no PrP on which to dock. (B) Shows two different PrP-res conformers induced by two different TSE viral strains (A and B particles with protective capsid cages). As previously modeled,Citation47 the infectious particle initiates or “seeds” the PrP misfolding. As now shown experimentally, this amyloid can continue to perpetuate itself even after agent elimination.Citation39 (C) Top depicts the manufacture of abundant TSE infectious particles with a residue of limited tightly bound PrP membrane attachment sites. Bottom shows late accumulating host PrP-res amyloid that can trap, protect, and eventually eliminate agent.Citation39
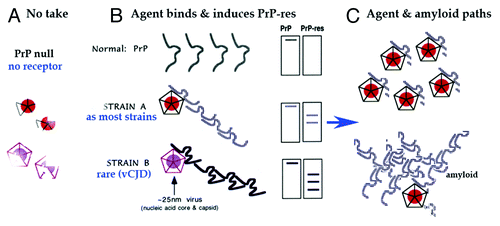
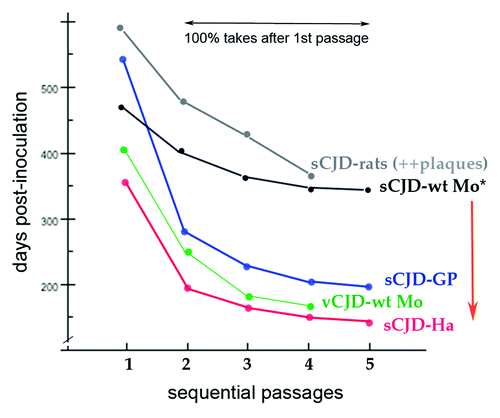
Figure 4. Composite published experimental data for transmission of sCJD human brain to different species in sequential passages. Guinea pig (GP), hamster (Ha), murine (Mo), and rat sCJD plots contain original data from the first serial transmission of a human TSE agent to these small animalsCitation23,Citation35,Citation70,Citation71 as well as additional transmissions. All show a significant reduction in incubation time in serial passages. The sCJD agent from human and from hamster brain homogenates give the identical disease with the same very prolonged incubation time.Citation31 Even after serial passages in standard wt mice with a prolonged incubation time, the sCJD agent (*) retains and immediately expresses its virulent rapid incubation mutation acquired during its first hamster adaptation (red arrow) as previously documented.Citation66 The sCJD agent in rats induces many plaques and widespread disease,Citation23 unlike the limited lesions provoked in mice.Citation35,Citation36 Thus plaque deposition is a species-specific reaction to an agent, and not necessarily a consequence of a long incubation time as sometimes assumed. Even after serial rat passages FU-CJD also retains its virulence for murine cells.Citation39 Note that human adapted vCJD (as BSE) does not require a human PrP sequence to infect mice; it rapidly adapts to mice, and here exhibits a very different incubation pattern than sCJD. The vCJD agent (and BSE) also induce the same distinctive regional neuropathology in mice.Citation22