Figures & data
Figure 1. The effect of cerebral malaria on the blood–brain barrier (BBB) and the developing neurovascular unit. The BBB can be separated into the physiological BBB, where the endothelial cells are in close apposition to astrocyte end processes and pericytes, and the neuroimmunological BBB, which has a perivascular space separating the endothelial cells from the astrocytic foot processes. During malaria infection, the endothelium becomes activated leading to both transcellular and paracellular leak. The neurovascular unit, which makes up the BBB, undergoes marked changes during development which may impact the pathogenesis of cerebral malaria, including: synaptic pruning, myelination, immune system maturation, maturation and differentiation of glial cells, increased expression of tight junction proteins, and ultimately improved endothelial barrier function.
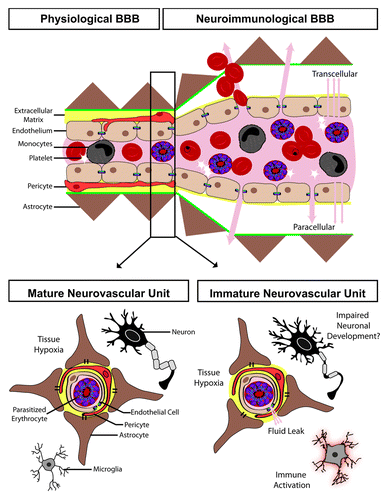
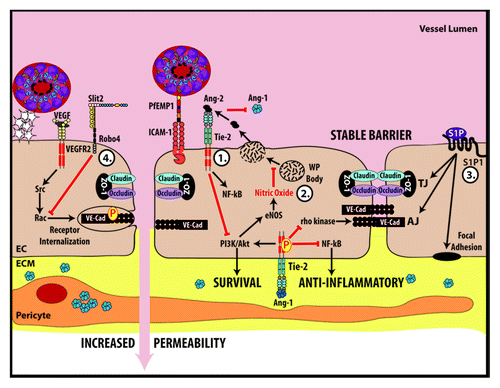
Figure 2. Key molecular pathways that govern endothelial barrier function and may play a role in the endothelial dysfunction occurring during malarial infection. (1) Ang-1 mediated signaling inhibits NFκB, prevents the internalization of VE-Cadherin, promotes nitric oxide formation and promotes endothelial cell survival. Conversely, Ang-2 competes with Ang-1 for Tie2 binding after being released during Weibel–Palade (WP) body exocytosis. Ang-2 antagonizes the effects of Ang-1. (2) Nitric oxide inhibits exocytosis of WP bodies. (3) Sphingosine-1-phosphate (S1P) stabilizes proteins that make up tight junctions, adherens junctions and focal adhesions. (4) Slit-Robo4 signaling promotes VE-cadherin localization to the plasma membrane and inhibits VEGF mediated endocytosis of VE-cadherin. EC, endothelial cell; ECM, extracellular matrix.