Figures & data
Figure 1. Interplay between reactive oxygen and reactive nitrogen species. The host enzyme NADPH oxidase (NOX) generates superoxide (O2•−) from O2. Aerobic metabolism within the pathogen inevitably results in side reactions in which successive one electron reductions of O2 yields the reactive oxygen species, O2•−, hydrogen peroxide (H2O2) and the hydroxyl radical (•OH). Nitric oxide (NO) is generated by the action of host inducible nitric oxide synthase (iNOS) (and by some bacteria that possess nitric oxide synthase). Nitric oxide is a reactive free radical and is a source of reactive nitrogen species such as nitroxyl (NO−), nitrosonium (NO+), and peroxynitrite (ONOO−), which is formed by reaction of NO with O2•−, or NO− and O2, and peroxynitrous acid (OONOH). Nitric oxide reacts with thiol groups to modify activity by the formation of S-nitrosylated proteins (RSNO). Nitric oxide can be detoxified by the flavohemoglobin Hmp by conversion to nitrate (NO3−) in the presence of O2. Some bacteria are capable of denitrification in which NO3− is converted to nitrogen gas (N2) via NO as an intermediate. In the absence of O2, the major detoxification mechanism in E. coli is the anaerobic NO reductase NorVW (O2-sensitive flavorubredoxin) that converts 2NO to N2O (nitrous oxide) and water. A similar reaction can be catalyzed by Hmp and NrfA in the absence of O2. Some terminal oxidases can also reduce NO to N2O, or by reaction of ferryl heme (FeCitation4+ = O2−) with NO generate NO2−.
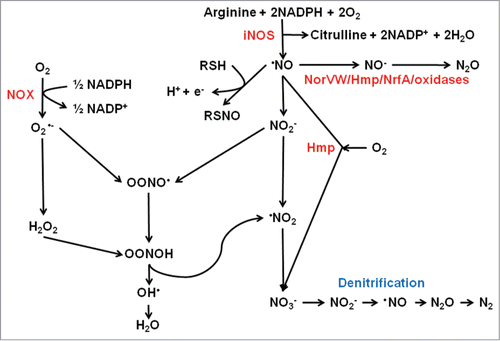
Figure 2. Scheme summarizing the changes in the FNR iron–sulfur cluster that occur upon reaction with O2 or NO and regulation of the hlyE gene. In E. coli, newly translated apo-FNR acquires a cubic [4Fe-4S] cluster (iron in red, cluster sulfur in yellow, Cys sulfur in orange) via the action of the iron sulfur cluster biosynthetic machinery (Isc). In the absence of O2, the [4Fe-4S] form of FNR is stable and cluster acquisition promotes dimerization and enhanced site-specific (consensus sequence: TTGATNNNNA TCAA) DNA-binding at target promoters, such as that encoding the cytolysin HlyE. Expression of hlyE is driven from a class I FNR-dependent promoter (FNR binding site located at −61.5 relative to the transcript start, +1) via interactions between the downstream subunit of FNR (blue oval with yellow cube) and the C-terminal domain of the α-subunit of RNA polymerase (brown). In the presence of oxygen (O2) the [4Fe-4S] cluster is converted to a planar [2Fe-2S] cluster via a [3Fe-4S] intermediate. This is accompanied by conversion of FNR from the DNA-binding competent dimeric form to the transcriptionally inactive monomer. During this process, cluster sulfide can be retained in the form of a persulfide-ligated [2Fe-2S] form of FNR, allowing facile repair of the cluster and a return to the [4Fe-4S] form. Prolonged exposure to O2 results in the breakdown of the [2Fe-2S] forms of the protein resulting in apo-FNR, which can acquire a [4Fe-4S] cluster by interaction with Isc. The FNR [4Fe-4S] cluster also reacts with NO yielding an octanitrosylated form and dinitrosyl iron complexes. Like O2, reaction with NO results in FNR inactivation.
![Figure 2. Scheme summarizing the changes in the FNR iron–sulfur cluster that occur upon reaction with O2 or NO and regulation of the hlyE gene. In E. coli, newly translated apo-FNR acquires a cubic [4Fe-4S] cluster (iron in red, cluster sulfur in yellow, Cys sulfur in orange) via the action of the iron sulfur cluster biosynthetic machinery (Isc). In the absence of O2, the [4Fe-4S] form of FNR is stable and cluster acquisition promotes dimerization and enhanced site-specific (consensus sequence: TTGATNNNNA TCAA) DNA-binding at target promoters, such as that encoding the cytolysin HlyE. Expression of hlyE is driven from a class I FNR-dependent promoter (FNR binding site located at −61.5 relative to the transcript start, +1) via interactions between the downstream subunit of FNR (blue oval with yellow cube) and the C-terminal domain of the α-subunit of RNA polymerase (brown). In the presence of oxygen (O2) the [4Fe-4S] cluster is converted to a planar [2Fe-2S] cluster via a [3Fe-4S] intermediate. This is accompanied by conversion of FNR from the DNA-binding competent dimeric form to the transcriptionally inactive monomer. During this process, cluster sulfide can be retained in the form of a persulfide-ligated [2Fe-2S] form of FNR, allowing facile repair of the cluster and a return to the [4Fe-4S] form. Prolonged exposure to O2 results in the breakdown of the [2Fe-2S] forms of the protein resulting in apo-FNR, which can acquire a [4Fe-4S] cluster by interaction with Isc. The FNR [4Fe-4S] cluster also reacts with NO yielding an octanitrosylated form and dinitrosyl iron complexes. Like O2, reaction with NO results in FNR inactivation.](/cms/asset/9d216ddf-4039-4651-aa72-4016cc2aea1e/kvir_a_981130_f0002_oc.gif)
Figure 3. Regulation of the P. aeruginosa T3SS. Genetic regulation of the P. aeruginosa T3SS is complex. The O2 sensor, ANR, activates T3SS gene expression indirectly (dashed lines) via activation of narL gene expression under low O2 conditions and subsequent effects of the regulatory RNAs, rgRsmY and rgRsmZ on expression of the regulator RsmA. The activity of the rgRsmY and rgRsmZ regulatory RNAs is also controlled by the RetS/LadS/GacS/GacA cascade. The two-component system GacS–GacA is required for virulence in many hosts and phosphorylated GacA activates expression of rgRsmY and rgRsmZ. GacS is inhibited by formation of heterodimers with RetS and LadS activates the GacS–GacA system by an as yet unknown mechanism. In the presence of high Ca(II) concentrations, ExsE sequesters the anti-anti-activator ExsC, permitting the anti-activator ExsD to interact with the activator ExsA. Consequently, expression of the 43 genes required for the function of the T3SS is not activated. In the presence of low Ca(II) concentrations, ExsE is secreted. As a result ExsC sequesters ExsD, releasing ExsA to activate genes encoding the T3SS. ExsA-mediated activation is also antagonized by the anti-activator PtrA, by PtrB via the RecA response to DNA damage, and by PsrA (in response to long chain fatty acids, LCFA). The alginate regulators (MucA, AlgT/U, AlgR) act to repress T3SS expression indirectly. In addition, the efflux pump regulator MexT controls T3SS gene expression via the action of PtrC. T3SS genes are represented by a single rectangle. Arrows indicate activation, T-junctions indicate repression, solid lines indicate direct regulation, and dashed lines indirect regulation.
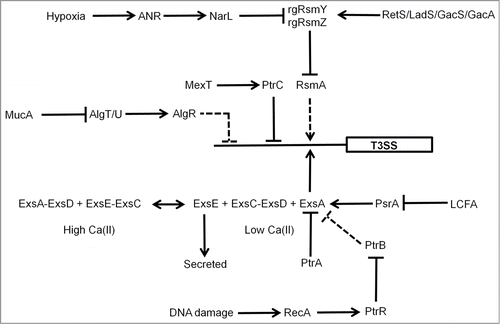
Figure 4. Scheme summarizing the action of NorR at the E. coli norVW promoter. (A) In the absence of NO hexameric NorR (unfilled ovals) is able to bind to enhancer elements located upstream of the norVW core σ54-dependent promoter elements (-12 and -24) via its helix-turn-helix (H-T-H) DNA-binding domain. Integration host factor (IHF) bends the DNA such that NorR and the σ54-RNA polymerase holoenzyme can potentially interact. However, these interactions are unproductive because the ATPase activity of the NorR AAA+ domain is inhibited by interaction with the GAF domain, which contains the sensory mononuclear iron center (Fe[II]) (see inset). Consequently, norVW transcription is switched off (small filled arrow, +1). (B) When NO binds at the mononuclear iron centers of NorR (Fe[II]-NO) the AAA+ domain is released from the sensory GAF domain (see inset) and acquires ATPase activity allowing productive interactions with σ54-RNA polymerase. The ensuing conformational changes promote the formation of the open complex and enhance norVW transcription (large filled arrow, +1). For clarity, not all the regulatory elements operating at this promoter are shown. The diagram is not drawn to scale.
![Figure 4. Scheme summarizing the action of NorR at the E. coli norVW promoter. (A) In the absence of NO hexameric NorR (unfilled ovals) is able to bind to enhancer elements located upstream of the norVW core σ54-dependent promoter elements (-12 and -24) via its helix-turn-helix (H-T-H) DNA-binding domain. Integration host factor (IHF) bends the DNA such that NorR and the σ54-RNA polymerase holoenzyme can potentially interact. However, these interactions are unproductive because the ATPase activity of the NorR AAA+ domain is inhibited by interaction with the GAF domain, which contains the sensory mononuclear iron center (Fe[II]) (see inset). Consequently, norVW transcription is switched off (small filled arrow, +1). (B) When NO binds at the mononuclear iron centers of NorR (Fe[II]-NO) the AAA+ domain is released from the sensory GAF domain (see inset) and acquires ATPase activity allowing productive interactions with σ54-RNA polymerase. The ensuing conformational changes promote the formation of the open complex and enhance norVW transcription (large filled arrow, +1). For clarity, not all the regulatory elements operating at this promoter are shown. The diagram is not drawn to scale.](/cms/asset/c6b87bea-7739-4615-9115-50544820cdcb/kvir_a_981130_f0004_oc.gif)
Figure 5. Scheme summarizing the redox-reactivity of OxyR. (A) The sensory C-terminal domain of each monomer in the OxyR homotetramer contains a redox-reactive cysteine residue (Cys-199), which forms a sulfenic acid (S-OH) in the presence of peroxide stress. This form of OxyR is proposed to be able to regulate gene expression, although more likely acts as an intermediate in forming the true active form, which is able to bind DNA and serve as a transcriptional regulator and contains an intra-molecular disulphide bond between Cys-199 and Cys-208. OxyR returns to its inactive form (Cys-199, SH; Cys-208, SH) by the action of glutaredoxin 1 and glutathione. (B) A secondary role of OxyR is as a nitrosative stress responder. S-nitrosylation of Cys-199, forming S-NO, leads to activation of OxyR, de-nitrosylation, forming SH, returns OxyR to its inactive form.
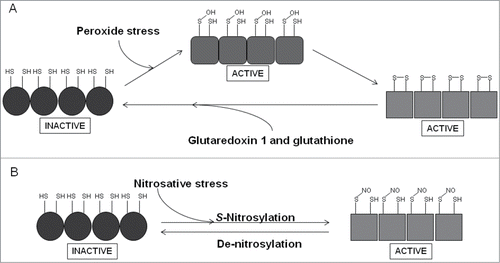
Figure 6. The Agr regulatory system. The agr locus consists of divergently transcribed agrBDCA and RNAIII genes. The former is driven from promoter 2 (P2) and encodes proteins that constitute the Agr quorum sensing system. The latter is driven from P3 and encodes the 26 amino acid δ-hemolysin and the regulatory RNA, RNAIII. AgrC and AgrA are a two-component system that responds to accumulation of an autoinducer peptide (AIP, a tailed thiolactone ring) that is generated by processing of AgrD by the membrane-bound AgrB protein and SpsB. The accumulation of AIP in the extracellular milieu is sensed by AgrC resulting in phosphorylation and activation of AgrA. RNAIII downregulates expression of cell surface proteins and upregulates exoprotein (toxin) production.
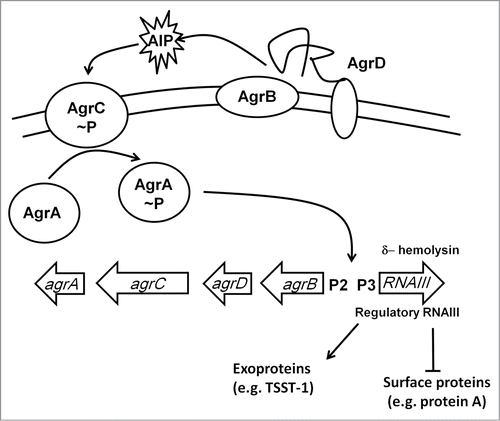
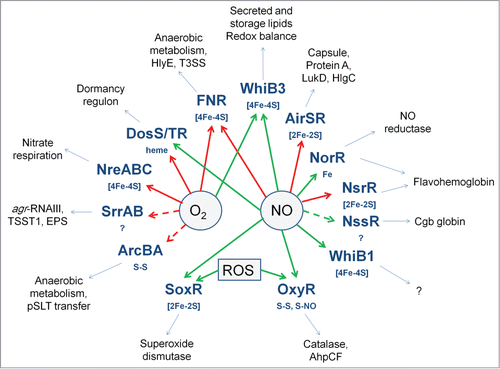
Figure 7. Oxygen- and NO-responsive bacterial transcription factors. The transcription factors with their sensory co-factors (if these are known) are shown in bold type-face. Direct sensing of O2 or NO is indicated by solid arrows; indirect or unknown sensing mechanisms by broken arrows; red arrows indicate that the signal molecule inhibits DNA-binding; green arrows indicate that the signal molecule promotes DNA-binding. Examples of virulence-related processes, toxins, cell-structural components, and proteins that are regulated by the transcription factors are shown in the outer ring.