Figures & data
Figure 1. Temperature-dependent changes in the membrane lipid composition of L. monocytogenes. Growth of L. monocytogenes EGD-e in BHI in the presence of [32P] PPi is represented as a measure of optical density by the black line. Aliquots of bacterial culture were removed and used for lipid isolation and subsequent separation by TLC. The percentage of each lipid species of the total lipid composition was determined by phosphorimaging. The percent of the total lipids made up of each species is relative to the area occupied on the graph. Detected lipids include cardiolipin (CL), lysylcardiolipin (LysCL), lysylphosphatidylglycerol (LysPG), phosphatidylglycerol (PG), unidentified lipid 1 (UL1), and unidentified lipid 2 (UL2). UL2 was present in the membranes of L. monocytogenes EGD-e grown at 37 °C (B), but not at 30 °C (A).
![Figure 1. Temperature-dependent changes in the membrane lipid composition of L. monocytogenes. Growth of L. monocytogenes EGD-e in BHI in the presence of [32P] PPi is represented as a measure of optical density by the black line. Aliquots of bacterial culture were removed and used for lipid isolation and subsequent separation by TLC. The percentage of each lipid species of the total lipid composition was determined by phosphorimaging. The percent of the total lipids made up of each species is relative to the area occupied on the graph. Detected lipids include cardiolipin (CL), lysylcardiolipin (LysCL), lysylphosphatidylglycerol (LysPG), phosphatidylglycerol (PG), unidentified lipid 1 (UL1), and unidentified lipid 2 (UL2). UL2 was present in the membranes of L. monocytogenes EGD-e grown at 37 °C (B), but not at 30 °C (A).](/cms/asset/7f4c1baa-803b-4c52-9496-4e7342cd8130/kvir_a_10928359_f0001.gif)
Figure 2. The effect of growth phase progression on lipid composition in B. subtilis. The growth of B. subtilis 1A100 at 37 °C in LB in the presence of [32P] PPi, is represented by the black line. Aliquots of bacterial culture were removed and used for lipid isolation and subsequent separation by TLC. The percentage of each lipid species of the total lipid composition was determined by phosphorimaging. Each lipid species found in the membrane of B. subtilis is represented, and the proportion of the membrane it occupies is reflected in the corresponding shaded area. The shading of the rows matches that of the area represented by the lipid species on the graph. Abbreviations are as follows: lysylphosphatidylglycerol (LysPG), phosphatidylglycerol (PG), cardiolipin (CL), and phosphatidylethanolamine (PE).
![Figure 2. The effect of growth phase progression on lipid composition in B. subtilis. The growth of B. subtilis 1A100 at 37 °C in LB in the presence of [32P] PPi, is represented by the black line. Aliquots of bacterial culture were removed and used for lipid isolation and subsequent separation by TLC. The percentage of each lipid species of the total lipid composition was determined by phosphorimaging. Each lipid species found in the membrane of B. subtilis is represented, and the proportion of the membrane it occupies is reflected in the corresponding shaded area. The shading of the rows matches that of the area represented by the lipid species on the graph. Abbreviations are as follows: lysylphosphatidylglycerol (LysPG), phosphatidylglycerol (PG), cardiolipin (CL), and phosphatidylethanolamine (PE).](/cms/asset/eb414d17-84c0-4421-8a39-fd7155f9e27c/kvir_a_10928359_f0002.gif)
Figure 3.Bacillus subtilis strain 1A100 ΔlysPGS is susceptible to β-lactam antibiotics. The presence of LysPG in the membrane allows B. subtilis 1A100 (red) to resist the effects of the cell wall targeting, β-lactam antibiotics azlocillin (green), and cefsulodin (pink). Concentrations of azlocillin and cefsulodin were used at the minimum inhibitory concentration of the ΔlysPGS strain corresponding to 50 μg/mL and 2.5 μg/mL, respectively. The absence of LysPG in B. subtilis 1A100 ΔlysPGS does not result in a growth defect in the absence of antibiotic (blue), but causes decreased growth compared with the wild type strain in the presence of azlocillin (black) and cefsulodin (purple). Data represent averages of three independent experiments and the corresponding standard errors.
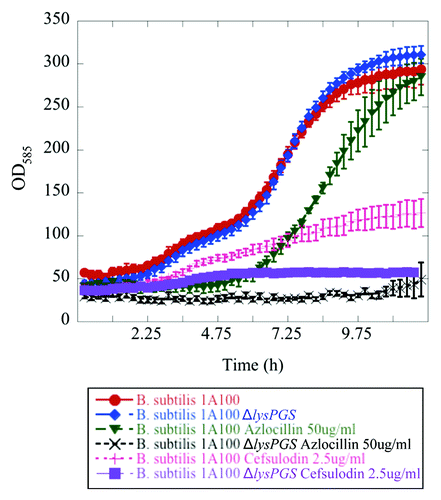
Table 1. Loss of LysPG results in impaired growth of L. monocytogenes in the presence of osmolytes
Figure 4. LysPG loss results in decreased survival of L. monocytogenes in the presence of bile. Colony forming units/mL remain the same for L. monocytogenes EGD-e (red) and L. monocytogenes EGD-e ΔlysPGS::lysPGS (black) after incubation in 24% bile over the course of 4 h, while the survival rate of L. monocytogenes EGD-e ΔlysPGS (blue) decreases as does that of positive control, L. monocytogenes EGD-e Δbsh (green). Data represent averages of three independent experiments and the corresponding standard errors.
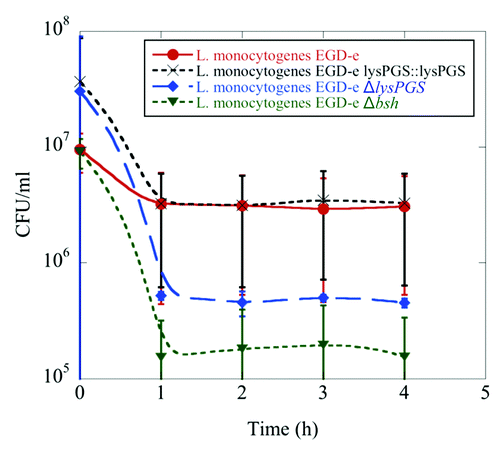
Figure 5. LysPG formation does not play a role in L. monocytogenes EGD-e resistance to the defensins RC-1 and HNP-1. No significant difference is observed in the percent proliferation of the ΔlysPGS strain (blue) in comparison to the wt strain (red) when incubated for 15 min in the presence of varying concentrations of RC-1 or HNP-1. Data was normalized to bacterial counts resulting from the incubation of both strains in DMEM alone, which was set to 100% proliferation. Calculated P values for the tested concentrations of RC-1 and HNP-1 are not less than 0.05, indicating no statistically significant difference between the percent proliferation of the two strains incubated in these conditions.
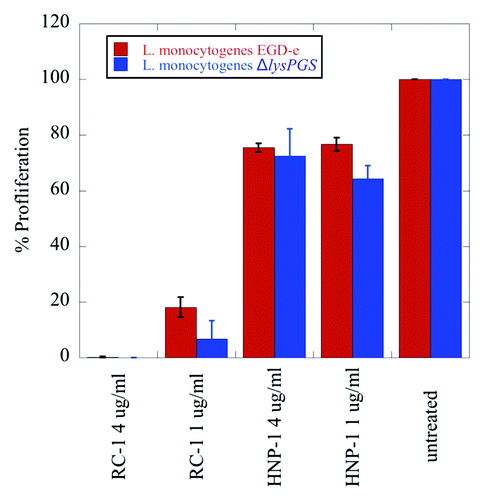
Figure 6. Changes in protein localization correlated with LysPG loss in B. subtilis and L. monocytogenes. Arrows indicate bands that were sent for identification by mass spectrometry and numbers indicate positive identifications in comparison to the appropriate control. Strains are abbreviated as follows: L. mo, L. monocytogenes; EGD-e, L. monocytogenes EGD-e; ΔlysPGS, L. monocytogenes EGD-e ΔlysPGS; B. su, B. subtilis; 1A100, B. subtilis 1A100; ΔlysPGS, B. subtilis 1A100 ΔlysPGS. Numbered excised bands were subsequently analyzed as shown in (S100 band 1) and (extracellular bands 1 and 2).
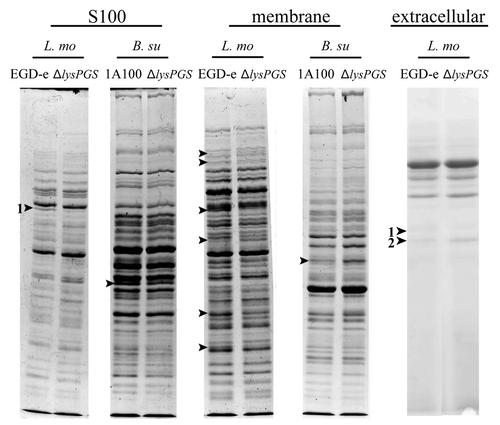
Table 2. Comparison of the cytosolic protein profile of L. monocytogenes EGD-e ΔlysPGS and the wild-type strain
Table 3. Comparison of the secreted protein profile of L. monocytogenes EGD-e ΔlysPGS and the wild-type strain
Figure 7. Loss of lysPGS does not affect L. monocytogenes adherence to or internalization by Caco-2 cells. Graphs show the number of bacteria associated per Caco-2 cell (A) or the percent of the total bacteria internalized (B) for the L. monocytogenes EGD-e (wt) or L. monocytogenes EGD-e ΔlysPGS (ΔlysPGS) after 10 min and 1 h following infection.
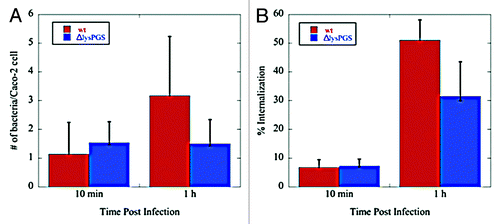
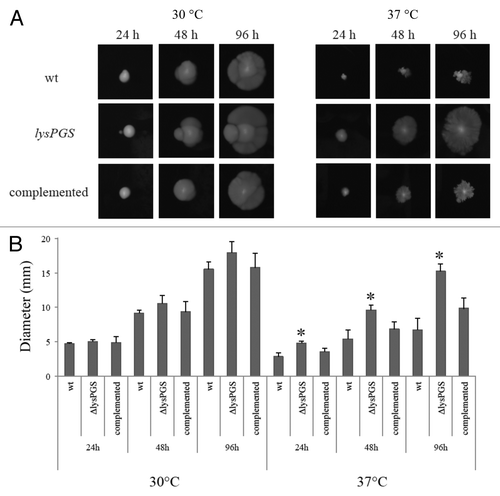
Figure 8. Loss of LysPG in the membrane allows L. monocytogenes EGD-e to remain motile at 37 °C. Colony diameters are similar for all three strains when grown at 30 °C. At 37 °C the L. monocytogenes EGD-e ΔlysPGS colony diameter is consistently larger than the colony diameters of both the L. monocytogenes EGD-e (wt) and L. monocytogenes EGD-e ΔlysPGS::lysPGS (complemented) strain (A). The assay was repeated in triplicate and plated on BHI agar in duplicate. Diameter averages are represented in graphical form with statistically relevant (two tailed t test values of P < 0.001) differences indicated by an asterisk (B).