Figures & data
Figure 1. Model for transcription regulation by PhoB and transmembrane signal transduction by environmental Pi and the Pst system in E. coli (adapted from refs. 5 and 56). Two processes are involved in Pho regulon control: inhibition when Pi is in excess and activation under Pi limitation. The Pst transport system is associated with the PhoR histidine kinase/phosphatase, which controls the phosphorylation state of the response regulator PhoB. At high Pi concentrations (>4 μM), PhoR might interact with the PstSCAB-PhoU complex, which represses its autophosphorylation and acts as a PhoB phosphatase. In contrast, low extracellular Pi concentrations sensed by Pst system, which increases the kinase activity of PhoR, resulting in the accumulation of phosphorylated PhoB∼P. Likewise, loss-of-function mutations in the pst genes lead to the constitutive phosphorylation of PhoB, regardless of the external Pi concentration. The Pi binding protein PstS acts as the primary sensor of external Pi concentration. When the Pi concentration is low (<4 μM), PhoR undergoes conformational change, resulting in its release from the repressor complex and autophosphorylation. PhoR is the histidine kinase/phosphatase that donates a phosphoryl group to PhoB when environmental Pi is limiting and removes the phosphoryl group from PhoB∼P when environmental Pi is abundant. PhoB is a typical winged-helix response regulator that upon aspartyl phosphorylation forms a dimer, which binds to DNA sequences upstream of Pho regulon genes to recruit RNA polymerase (RNAP) and initiate transcription. In some cases the transcription is repressed possibly by disruption of RNAP interaction with other activators.
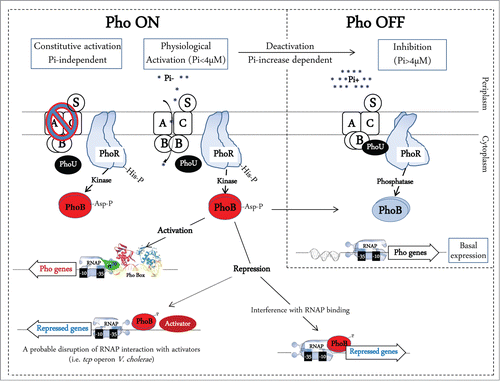
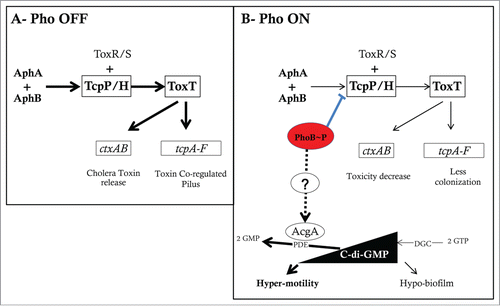
Figure 2. The V. cholerae virulence cascade and the mechanisms of Pi/Pho regulation of virulence, motility, and biofilm formation. (A) V. cholerae virulence cascade. In high Pi conditions (Pho OFF) and in conditions favoring gene expression of AphA and AphB regulators, AphA cooperates with AphB to activate the tcpPH promoter. TcpPH cooperatively with ToxR/S activates the toxT promoter. ToxT then activates the tcpA-F and ctxAB promoters. This increases colonization through formation of the toxin co-regulated pilus (TCP) and cytotoxicity through Cholera toxin (CT) release. (B) In low Pi conditions (or Δpst mutation), PhoB∼P binds to the Pho-box located between the tcpPH promoter and the binding sites for AphA and AphB. This results in tcpPH repression which reduces the downstream ToxT-dependent virulence cascade. C-di-GMP is synthesized from 2 GTP molecules by diguanylate cyclases (DGCs) and degraded to 2 GMP molecules by phosphodiesterases (PDEs). Studies in V. cholerae have shown that c-di-GMP increases biofilm formation and reduces the motility and toxT expression. In low Pi (or Δpst mutation) PhoB induces indirectly the expression of acgAB which results in degradation of c-di-GMP leading to an increase in motility and a decrease of biofilm formation.