Figures & data
Figure 1. Surface display of the Gaussia princeps luciferase on Aspergillus fumigatus. (A) Schematic presentation of the integrative plasmid to express a synthetic, codon-optimized version of the gluc gene in A. fumigatus and to target its product to the cell surface by anchoring it to the membrane protein MP1. Functional elements of the expression cassette are the constitutively transcribed gpdA promoter (pgpdA) from A. nidulans and the his2A terminator region (his2At) from A. fumigatus. For secretion, the GLuc coding sequence (gluc) is preceded by a signal sequence (ss) from the A. oryzae tglA gene and the Rhyzopus oryzae N28 region. In-frame fusion via a (GA)5 linker region to the coding region of the GPI-anchored MP1 protein from A. fumigatus results in surface display of the bioluminescent reporter. (B) X-ray film autoradiography after addition of the GLuc substrate coelenterazine to mycelia of different transformants carrying the expression construct. Besides the wild-type recipient strain ATCC 46645, isolates carrying one, two and four copies, respectively, of the integrative plasmid pSK481 are displayed. (C) Light intensities emitted from GLuc reporter strains expressing varying doses of the bioluminescent reporter based on the indicated copy numbers. Photographs were taken before (upper panel) and after (lower panel) coelenterazine addition with a digital camera in the light and dark, respectively.
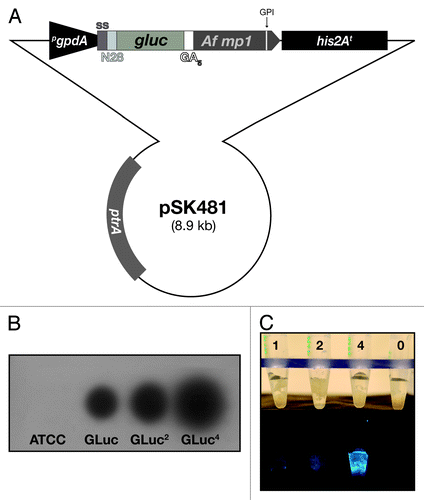
Figure 2. Exposure of the bioluminescent reporter GLuc on the surface of A. fumigatus AfS75. (A) Indirect immunofluorescence with a monoclonal antibody against the G. princeps luc gene product displays association of the fluorescent signal with the hyphal surface. (B) Partial tryptic digest of AfS75 germlings results in a decrease of bioluminescence emitted from this strain. Shown are photon fluxes derived from 1 × 108 germlings that had been digested with trypsin for the indicated periods of time.
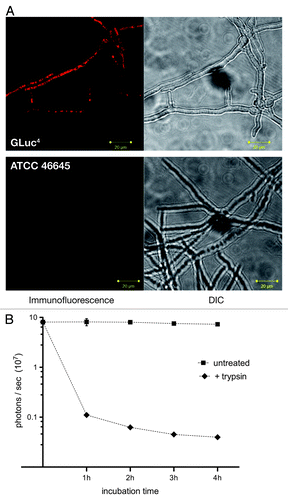
Figure 3. Bioluminescent properties of the reporter strain expressing surface-exposed GLuc. (A) AfS75 displays a flash kinetic of the bioluminescent signal. Emission of photons after substrate addition to 1 × 106 germinated conidia was monitored for approximately six minutes to result in a rapid, nonlinear decay of luminescence. (B) Bioluminescence can be detected from fungal structures formed from at least 1,000 conidia as early as 4 h. Increased spore numbers or extended incubation times result in increases of signal intensities. Subtracted background values from blank medium were in a range of 1–2 × 102 photons per second.
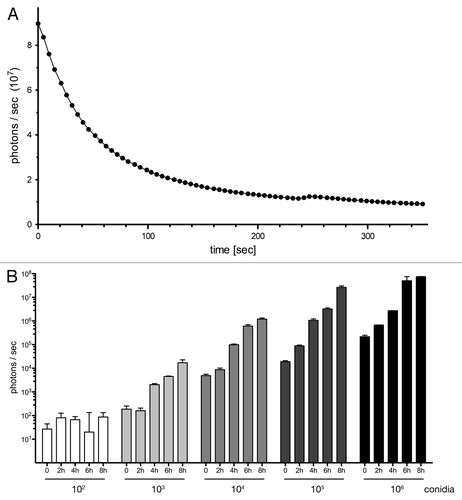
Figure 4. Longitudinal study of cutaneous aspergillosis in neutropenic and immune competent BALB/c mice. (A) Animals were infected subcutaneously with 5 × 105 conidia of bioluminescent strain AfS75 (GLuc4) or its wild-type progenitor ATCC 46645 (ATCC) at the contralateral side, and emission of photons after injection of the G. princeps luciferase substrate coelenterazine was followed for one minute up to seven days post-infection (dpi). Bioluminescent signals manifested only from the AfS75-infected areas, whereas the ATCC strain did not result in any background luminescence. Variations in signal intensity ranges were evident as indicated by the differing scales of luminescence. Immune competent animals were able to completely clear the infection within several days. (B) Histopathologic GMS specimen (100x) of representative dorsal tissue after three days post-infection with 5 × 105 conidia of AfS75, demonstrating subcutaneous localization of a hyphal cluster. (C) Fungal burdens increased over the course of cutaneous infection as estimated by corrected galactomannan indices (cGMIs); however, no statistically significant correlation of these to bioluminescence signal intensities could be deduced (not shown).
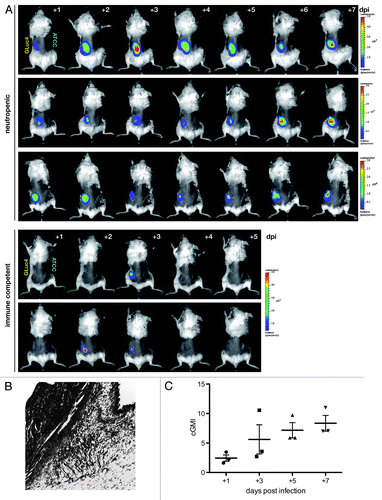
Figure 5. Non-invasive imaging of bioluminescence with increasing amounts of fungal spores. (A) Neutropenic animals were infected with the indicated numbers of conidia from reporter strain AfS75 and luminescent signals were monitored at the indicated time points post-infection. (B) Photon fluxes emitted from infected regions correlate to the initial infection doses, as determined from signal intensities sampled from two to three animals eight hours after infection.
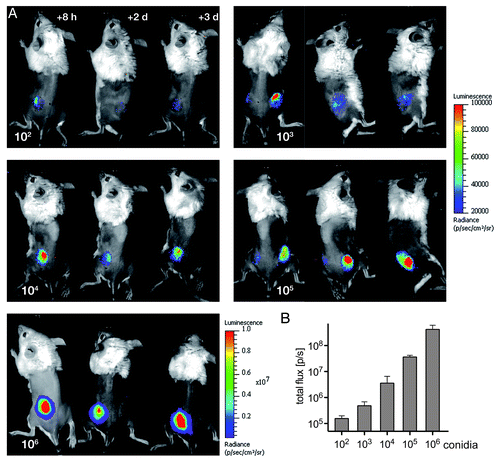
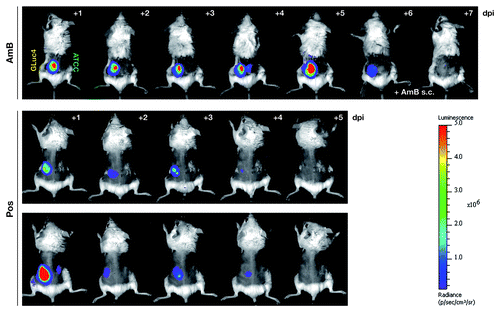
Figure 6. The bioluminescent reporter strain AfS75 allows in vivo monitoring of antifungal therapy. Shown are animals from an in vivo imaging series of neutropenic mice infected with 5 × 105 conidia of AfS75 (GLuc4) and the wild-type isolate ATCC 46645 (ATCC), respectively, that had been treated with amphothericin B deoxycholate (AmB, 5 mg/kg/day i.p.) or posaconazole (40 mg/kg/day per os), demonstrating failure of antifungal treatment by AmB or complete clearance of the fungal infection by posaconazole as evident from the persistence and absence, respectively, of bioluminescence. When applying AmB directly at the seat of infection (+ AmB s.c.), bioluminescence was resolved within two days.