Abstract
Introduction
Varicocele is defined as a pathological alteration in the venous circulation of the testis which appears almost exclusively on the left side.
The aim of current work was to compare the normal structure of the wall of the veins of the pampiniform plexus and also to highlight the occurrence of any structural alterations in these veins in cases of primary varicocele through a combined histopathologic and immunohistochemical study.
Methods
Histopathological examination and immunohistochemical studies using monoclonal antibodies against CD31 (a marker for endothelial cells) and neurofilament-200 (NF-200) (nerve fiber marker) were performed on spermatic vein fragments of 30 varicocele patients and 30 normal spermatic veins obtained from patients undergoing inguinal herniectomy.
Results
Normal spermatic veins showed an inner circular smooth muscle (SM) layer in the tunica media, and an outer longitudinal SM layer in the tunica adventitia. Grade I varicocele cases, showed an increase in the connective tissue of the adventitia and of the tunica media, which increased proportional to the degree of varicocele. The outer longitudinal smooth muscle layer of the large spermatic veins was significantly degraded in the presence of varicocele grades I and II, and did not even exist in varicocele grade III. Immunostaining for CD31 and NF-200 revealed that vasa vasorum and nerve fibers were decreased in the wall of large spermatic veins in patients with grades I and II varicocele, and were minimal to absent in patients with grade III varicocele.
Conclusion
The wall of pampiniform plexus veins is a complex structure of smooth muscle organization, inner circular and outer longitudinal fibers which is important for providing an effective blood transport through the pampiniform plexus. This mechanism is obviously damaged in varicocele patients.
1 Introduction
Varicocele is a pathologic dilatation of the pampiniform venous plexus of the spermatic cord that occurs in approximately 15–20% of males and in up to 40% of infertile males.Citation1,Citation2 This condition occurs most frequently on the left side and can cause decreased testicular function.Citation2 Some patients may have scrotal pain and swelling, but more importantly, varicocele is considered to be a potential cause of male infertility.Citation3 This relationship is controversial, but improved fertility and sperm quality have been reported after treatment, including occlusive treatment for varicoceles.Citation4,Citation5 On physical examination, large varicoceles are easily identified as the classic “bag of worms” surrounding the testis. Also the dilatation of spermatic veins can be assessed by the Valsalva maneuver. Ultrasonography, particularly Doppler ultrasonography, allows accurate diagnosis of varicoceles, even subclinical varicoceles.Citation6–Citation8
There are several theories on the etiology of varicocele, ranging from anatomic reasons, including the angle at which the left testicular vein enters the left renal vein, to functional deficiencies regarding the lack of valves at the junction of the testicular vein and renal vein.Citation9–Citation12 Despite the existing studies and ongoing research, the mechanisms of varicocele development remain controversial and are not fully understood. Recently, Tilki et al.Citation13 suggested that an antireflux mechanism can be due to the smooth muscle (SM) organization of spermatic veins, consisting of an outer longitudinal layer, an inner circular layer, and fibers branching from the outer to the inner layer.
Only few studies evaluated the structure of spermatic veins in varicocele patients,Citation3,Citation13,Citation14 and their results were controversial. Several authors described a thickening of the SM layer and extracellular matrix, mainly in patients with grade III varicocele,Citation3,Citation14 while others identified progressive degradation of the outer longitudinal SM layer, mainly in the tunica adventitia.Citation13
1.1 Aim of the work
Considering the inconsistency of available data, the aim of this work was to compare the normal anatomic structure of the veins of the pampiniform plexus and to assess any possible morphologic alterations in patients with varicocele through a combined histopathologic and immuno-histochemical study. Also, we aimed to assess whether these alterations might serve as a base for an explanation for the development of varicocele.
1.2 Materials
The present study was conducted prospectively on 30 patients undergoing varicocelectomy for idiopathic left and or right sided varicocele, (six patients with varicocele grade I, six patients with varicocele grade II, and 18 patients with varicocele grade III). The patients’ age ranged between 23 and 35 years. This study also included a control group of 30 subjects with no varicocele (proven both clinically and by ultrasonographic examination) admitted to the Department of General surgery, Alexandria Faculty of Medicine, for inguinal herniectomy.
Both patients and control subjects were treated at Main University Hospital, Faculty of Medicine, Alexandria University from January 2009 to December 2009. The biopsy specimens were obtained from both groups after signing an informed consent form, and the study was approved by the Alexandria Faculty of Medicine, Research Ethics Committee.
2 Methods
Preoperatively, all patients underwent physical examination, color-Doppler ultrasound and a semen analysis. This last examination was performed according to the WHO recommendations.Citation15 Upon physical examination, varicocele was classified according to Dubin and Amelar,Citation16 into grade I: varicocele detected during Valsalva maneuver; grade II: varicocele palpable in basal condition, and grade III: varicocele visible during physical examination. In addition, venous reflux was proven by CW-Doppler sonography in each patient to confirm the clinical diagnosis. Specifically, once having isolated a vein of the pampiniform plexus, a tract 2 to 3-cm long was dissected during varicocelectomy. To study the pampiniform plexus veins of patients without varicocele, we dissected a 2 to 3-cm tract of spermatic veins in 30 patients undergoing inguinal herniectomy (after signing an informed written consent form). In those cases, the presence of varicocele had been excluded by both physical examination and color-Doppler ultrasound. The dissected veins had the same distance from the testis in both groups.
Specimens obtained for conventional histopathologic (H&E) and subsequent immunohistochemical studies were immediately fixed in 10% neutral buffered formalin and processed to formalin fixed paraffin embedded tissue (FFPET) blocks.
2.1 Histopathologic examination
To analyse the vascular structure by light microscopy, in the 30 cases of normal spermatic cord tissue and 30 cases of fragments of spermatic veins of varicocele patients, we stained 5 μ thick cut sections with conventional H&E stain. Since varicocele veins, in particular, exhibited big differences in diameter within the same vein, thus, on the basis of the presence or absence of the outer smooth muscle layer, the spermatic cord veins were subclassified into two groups: large veins presenting an outer smooth muscle layer and small veins without such a layer.
2.2 Immunohistochemistry
To determine whether there are differences between normal spermatic veins and varicocele veins regarding vasa vasorum and nerve supply of the vascular wall, we performed immunohistochemical studies using antibodies against CD31 and neurofilament-200 on five micron-thick sections, prepared from FFPE tissue blocks that were cut and mounted on coated slides. Particularly the vascularisation and the nerve supply of the large veins presenting an outer smooth muscle layer were compared. The sections were deparaffinized in xylene and rehydrated in descending ethanol grades. Sections were incubated for 10 min in 3% hydrogen peroxide to block endogenous tissue peroxidase. For neurofilament-200 immunostaining no specific pre-treatment was required, whereas, for CD31 heat retrieval was done in microwave oven using EDTA 1 mM (pH 8.0) for 10 min. Tissue sections were immunostained for both CD31 and neurofilament-200 using prediluted monoclonal antibodies clones [JC/70A and 2F11 respectively], (NeoMarkers, Fremont, CA). The antigen-antibody reaction was visualized by Thermo scientific UltraVision LP Detection System. Immunohistochemical reactions were developed with diaminobenzidine and sections counterstained with Harrris hematoxylin. All immunostains were manually processed, with appropriate positive and negative controls (vascular tumor for CD31 and brain tissue for NF 200) included for each batch of slides.
2.3 Statistical analysis
Data were analyzed using SPSS software version 9.0. Results are given as median (min-max) for age and thickness of the wall of the spermatic veins and as number (%) for all other parameters.
3 Results
3.1 Clinical results
Median age of the 30 patients with varicocele was 29 years (i.r. 23–35) and of the 30 patients undergoing inguinal herniectomy was 28 years (i.r. 22–34). Varicocele was grade I in 6 patients (20%), grade II in 6 (20%), and grade III in 18 (60%) patients. As regards the affected side 27 patients (90%) had varicocele on the left side and three patients (10%) had varicocele bilaterally. The indication for treatment was semen analysis impairment in all patients (three semen analysis separated by one month).
3.2 Histopathological findings (H&E)
Histopathologic examination of the wall of normal spermatic veins with a large diameter showed a tunica intima consisting of endothelial cells overlying a subendothelial layer. The tunica media showed a continuous circular smooth muscle layer, separated by scarce connective tissue. The median thickness of the tunica media of normal spermatic veins as measured by occular micrometer was 125 μ (i.r. 96–140). The adventitia was well-identifiable and presented vasa vasorum and bundles of longitudinal smooth muscle cells, separated by more connective tissue than in the tunica media. The median adventitial thickness was 100 μ (i.r. 80–110). (a)
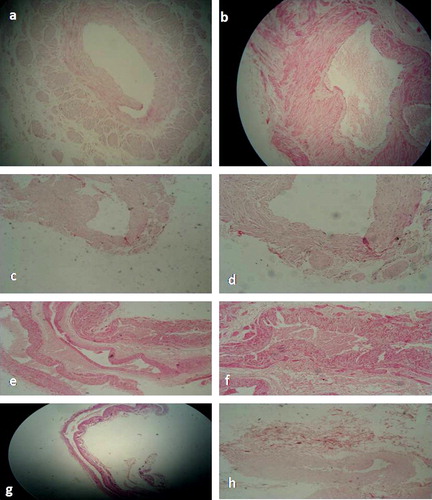
Small spermatic veins were classically structured with no additional smooth muscle layer in the adventitia.
Examination of the H&E stained sections of the wall of large spermatic veins in patients with varicocele revealed an unaltered intimal layer similar to that of the control group featuring no histopathologic abnormalities. Median thickness of the tunica media and tunica adventitia in patients with varicocele was 165 μ (i.r. 130–200), and 160 μ (i.r. 102–189) respectively, which is higher than the control group. These changes were related proportionally to the grade of varicocele. Also degradation or absence of the outer adventitial longitudinal smooth muscle layer in varicocele veins related proportionally to the degree of varicocele testis. Whereas in H&E stained sections, the reduction of the spermatic venous wall thickness in varicocele grade I resulted primarily from a degradation of the outer smooth muscle layer, yet, both smooth muscle layers were affected in severe (high grade) changes (b–h).
3.3 Vascularization and innervation of the pampiniform venous plexus
To study the vascularisation and innervation pattern of the wall of spermatic veins, we performed immunostaining for the endothelial cell marker CD31 and nerve fiber marker NF-200. Semiquantitative evaluation of CD31 and NF-200 immunostaining was done by the pathologist who was blinded to both clinical and sonographic findings (i.e.without knowledge concerning the group to which a case belongs as well as the grade of varicocele). Both CD31 and NF-200 immunostaining revealed higher numbers of vasa vasorum and nerve fibers respectively within the longitudinal smooth muscle layer in the adventitia of the wall of normal large spermatic veins of the control group compared to the varicocele group (a–c).
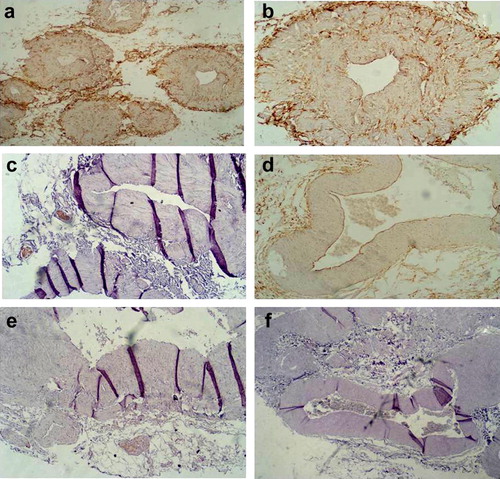
As regards large spermatic veins within the varicocele group, the vasa vasorum and nerve fiber counts were significantly reduced in varicocele veins (slightly lower in grade I, markedly reduced in grade II varicocele, and nearly absent in grade III varicocele) when compared to normal large sized spermatic veins. Thus, it became evident that the innervation and the vascularization of the tunica adventitia of the large spermatic veins decrease proportionate to the degree of varicocele as well as degradation of the outer longitudinal smooth muscle coat (d–f).
4 Discussion
Varicocele develops as a result of dilatation and tortuosity of veins of the pampiniform plexus secondary to retrograde flow into the internal spermatic vein (ISV). Varicocele is considered to be a potential cause of male infertilityCitation1–Citation3 and although this relationship is controversial, yet, improved fertility and sperm quality have been reported after varicocele treatment.Citation8,Citation11,Citation12,Citation16 The mechanism of varicocele development is still yet a subject of debate.Citation2,Citation3,Citation9,Citation13,Citation14
To date there have been only a few studies on the microscopic structure and histology of normal spermatic veins and varicocele veins. The pampiniform venous plexus consists of veins with different diameters and different vein wall anatomy.Citation17–Citation19 However, there is no detailed analysis considering these differences in spermatic vein histology and comparing them with varicocele histology.
This study aimed at comparing the normal histologic structure of the veins of the pampiniform plexus and assessing any possible morphologic alterations in patients with varicocele. Also, we aimed to assess whether these alterations might explain mechanisms underlying the development of varicocele.
The current study was done on 30 patients with right and or left sided varicocele of different grades and 30 control cases with inguinal hernia (varicocele was excluded both clinically and by Doppler ultrasonography). A segment of spermatic veins was taken from both groups and subjected to histopathological examination and immunohistochemical studies.
In the current study, histopathologic examination of pampiniform plexus veins of the control group revealed that the structure of large spermatic veins – which are the veins responsible for the main venous backflow into the testicular and renal vein – demonstrates a layer of circular SM cells within the tunica media and another longitudinally arranged SM bundles in the adventitia, which was thinner than that in the media. In opposition, the wall of small spermatic veins showed no outer longitudinal smooth muscle fibers in the tunica adventatia in both varicocele and control subjects, however, these veins are not the ones primarily involved in varicocele.
The structure of normal spermatic vein wall has been evaluated only in few studies.Citation13,Citation14 The wall of normal veins was reported by Tanji et al.Citation14 to be thin and constituted by only few SM cells with rich connective tissue. The SM fibers were shown to be primarily circular in configuration, and loosely arranged.Citation14 More recently, in a larger study involving patients undergoing orchiectomy for prostate cancer, Tilki et al.Citation13 reported that the normal venous wall shows a longitudinal SM layer within the adventitia and a circular layer within the media. For the first time, Tilki et al.Citation13 described in the serial sections the presence of oblique SM cells branching from the outer longitudinal to the inner circular layer. In the current study, our data augment the findings of Tilki et al.Citation13 using detailed and reproducible morphologic methods, in a more appropriate population of patients (age similar in control group to varicocele subjects).
In opposition to the control group, histopathologic examination of the wall of large spermatic veins showed an increase in the connective tissue within the adventitia and a progressive reduction of the outer longitudinal smooth muscle bundles, that went parallel with increasing severity of varicocele. It is noteworthy that in grade I varicocele an increase in the thickness of the tunica media together with an increase in the adventitia were found, suggesting that a compensatory mechanism (moderate hypertrophy of SM cells in the tunica media compensating SM loss of the adventitia) has been developed. The connective tissue increases in grades II and III and these differences are significant for grade III varicocele.
However Tanij et al.Citation14 described a thickening of venous wall in varicocele patients, mainly due to the increase in SM cells and extracellular matrix. These findings were most evident in grade III varicocele. The evaluation of venous wall with an electron microscope allowed the identification of the presence of an inner and an outer muscle layer, visible in grade II, more evident in grade III varicocele but absent in normal veins. The authors hypothesized that these changes occur to tolerate against increasing intraluminal pressure due to the blood reflux.Citation14 A thickening of the SM layer was also reported by Lee et al.Citation3 in patients with varicocele. Vice versa, Tilki et al.Citation13 observed a significant reduction of the longitudinal fibers, and such reduction was as high as 30–40% in grade I, 45–60% in grade II, and 70–80% in grade III varicocele. Moreover, those authors described for the first time the reduction up to disappearance of the oblique fibers with the increasing severity of the reflux, while a significant reduction of the number of circular muscle cells was recorded only in the cases with severe varicocele.Citation13
The discrepancies among the different authors might be ascribed to the different levels of dissection of spermatic veins performed to retrieve the segments: suprainguinal,Citation14 inguinal,Citation3 and subinguinal spermatic veins.Citation13
As regards immunohistochemical analyses the present study highlights the presence of a high number of vasa vasorum and nerve fibers within the adventitia of large sized spermatic veins of control subjects, which is in accordance with a previous report.Citation13 Moreover, our study in accordance with Tilki et al.Citation13 revealed a progressive reduction in the number of both vasa vasorum and nerve fibers in the wall of large spermatic veins in patients with varicocele, with no major alteration in the intima when compared to the control group.
Two pathophysiological hypotheses can be drawn according to the morphological findings described in the different studies. The increase in the pressure within the spermatic veins would cause the mechanical distension of the venous wall and the release of endothelial mediators, which might cause the increase in the number of SM cells and the deposition of extracellular matrix, which could ultimately cause an alteration of blood flow and reflux.Citation14 According to the data reported by Tilki et al.Citation13 and confirmed in our study, indeed, the alterations in the complex muscle structure might be caused by hypoxia due to the reduction in the number of vasa vasorum. The presence of hypoxia within the internal spermatic veins of varicocele patients has been recently reported by Lee et al.Citation3 who demonstrated the over expression of hypoxia-inducible factor-1α (HIF-1α). HIF-1α can stimulate the production of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-alpha (TGF-alpha) with the latter possibly being the main factor responsible for the increase in the fibrotic tissue of the venous wall in response to hypoxia.Citation20–Citation22 Further specific experimental studies are needed to confirm these hypotheses.
The distinct amount of blood vessels and nerve fibers in the outer smooth muscle layer according to Tilki et al.Citation13 probably serves to allow adequate contraction of the smooth muscles. Thus, decrease of vasculature and nerves (vasa vasorum and vasa nervosa) in varicocele might be associated with the degraded longitudinal smooth muscle layer with resultant loss of contractile function. Whether the degradation of the smooth muscle layer or the reduction in vasculature and nerve fibers comes first cannot be decided at this time.Citation13
The findings of this study, highlight the need for additional larger studies on both groups – normal and varicocele spermatic veins – to determine whether the degradation of the outer smooth muscle layer, decreased vasa vasorum and nerve fiber density in varicocele veins correlate with each other and or with the grade of varicocele testis.
The current study demonstrated altered anatomic architecture, vascularization as well as innervation in all studied venous fragments from varicocele veins. Whether this results in venous insufficiency with subsequent venous reflux – which is an important sign in the clinical diagnosis of varicocele testisCitation23,Citation24 – need further studies to be verified as at this time it is still not clear whether such structural alterations are a cause or a consequence of varicocele.
Also, in the current study, the age of the patients in both varicocele and non-varicocele was almost close, which did not allow us to test whether morphologic alterations do occur within normal spermatic veins with increasing age. However, this aspect was previously demonstrated by Tilki et al.Citation13 in their study, as they assumed that the age difference between their studied normal population and varicocele patients probably had no influence on the observed structural particularities, since the vein structure of varicocele grade I is more similar to normal structure than varicocele II and III. In addition, they also reported no changes between the normal veins of the younger and the normal veins of persons with older age in their study. Taken together these observations may therefore suggest that the outer smooth muscle layer is an essential structural component of the spermatic cord vein wall and does not change substantially in older age.Citation13
Our results show marked reduction in varicocele vein vascularization and innervations, in addition to degradation of the outer longitudinal smooth muscle coat. Whether a primary disturbance of the angiogenic vascularisation (vasa vasorum) or innervation of the vein wall is involved in these anatomic changes of the varicocele veins needs further verification.
5 Conclusions
The current study demonstrated that an outer adventitial longitudinal smooth muscle layer exists in addition to the normal circular smooth muscle layer of the tunica media in normal large spermatic veins. This paticular vein wall structure which simulates that of the veins of the lower leg or foot contributes to the venous transport mechanism in the pampiniform plexus, which is characteristically, exposed to a high hydrostatic pressure. This contractile function becomes disturbed in varicoceles by morphologic changes of the venous wall that may lead to impairment of blood return of the veins, promoting the development of varicocele.
Notes
Available online 3 September 2011
Peer review under responsibility of Alexandria University Faculty of Medicine
References
- T.B.HargreaveVaricocele – a clinical enigmaBr J Urol721993401408
- C.K.NaughtonA.K.NangiaA.AgarwalPathophysiology of varicoceles in male infertilityHum Reprod Update72001473481
- J.D.LeeS.Y.JengT.H.LeeIncreased expression of hypoxia inducible factor-1alpha in the internal spermatic vein of patients with varicoceleJ Urol175200610451048
- J.LeeS.BinsalehK.LoK.JarviVaricoceles: the diagnostic dilemmaJ Androl2922008143146
- P.KumanovR.N.RobevaA.TomovaAdolescent varicocele: who is at risk?Pediatrics12112008e53e57
- S.ResimM.CekA.FazliogluEcho-colour Doppler ultrasonography in the diagnosis of varicoceleInt Urol Nephrol3131999371382
- A.I.TasçiS.ResimT.CaskurluColor Doppler ultrasonography and spectral analysis of venous flow in diagnosis of varicoceleEur Urol3932001316321
- F.CornudX.BelinE.AmarVaricocele: strategies in diagnosis and treatmentEur Radiol931999536545
- H.U.BraedelJ.SteffensM.ZieglerM.S.PolskyM.L.PlattA possible ontogenic etiology for idiopathic left varicoceleJ Urol15119946266
- G.R.DohleG.M.ColpiT.B.HargreaveG.K.PappA.JungwirthW.WeidnerEAU guidelines on male infertilityEur Urol482005703711
- V.FicarraM.A.CerrutoG.LiguoriTreatment of varicocele in subfertile men: the Cochrane review—a contrary opinionEur Urol492006258263
- J.P.HeatonVaricocelectomy, evidence-based medicine and fallibilityEur Urol492006217219
- D.TilkiE.KilicR.TauberD.PfeiVerC.G.StiefR.TauberS.ErgunThe complex structure of the smooth muscle layer of spermatic veins and its potential role in the development of varicocele testisEur Urol515200714021409
- N.TanjiT.FujiwaraH.KajiS.NishioM.YokoyamaHistologic evaluation of spermatic veins in patients with varicoceleInt J Urol671999355360
- World Health Organization (WHO). WHO laboratory manual for the examination of human semen and sperm–cervical mucus interaction. 3rd ed. Cambridge: Cambridge University Press; 1999.
- L.DubinR.D.AmelarVaricocelectomy: 986 cases in a twelve-year studyUrology1051977446449
- S.ErgunN.KilicS.HarneitMicrocirculation and the vascular control of the testisAdv Exp Med Biol4241997163180
- N.KilicS.ErgunMethods to evaluate the formation and stabilization of blood vessels and their role in tumor growth and metastasis, Metastasis research protocols2001Humana PressOxford
- S.ErgunT.BrunsA.SoykaR.TauberAngioarchitecture of the human spermatic cordCell Tissue Res2881997391398
- M.OhhC.W.ParkM.IvanM.A.HoVmanT.Y.KimL.E.HuangN.PavletichV.ChauW.G.KaelinUbiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau proteinNat Cell Biol272000423427
- V.FalangaL.ZhouT.YuWtLow oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF-beta1J Cell Physiol191120024250
- M.ZimmerD.DoucetteN.SiddiquiO.IliopoulosInhibition of hypoxia-inducible factor is suficient for growth suppression of VHL/tumorsMol Cancer Res2220048995
- M.M.WishahiAnatomy of the venous drainage of the human testis: testicular vein cast, microdissection and radiographic demonstration. A new anatomical conceptEur Urol2021991154160
- M.M.WishahiDetailed anatomy of the internal spermatic vein and the ovarian vein. Human cadaver study and operative spermatic venography: clinical aspectsJ Urol14541991780784