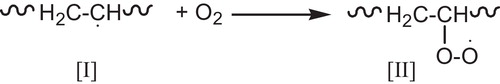Abstract
Polymer science is, of course, driven by the desire to produce new materials for new applications. The success of materials such as polyethylene, polypropylene, poly(vinyl chloride) and polystyrene is such that these materials are manufactured on a huge scale and are indeed ubiquitous.
1 Introduction
All commercial organic polymers will degrade in air when exposed to sunlight, although there is a very wide range of photo-oxidative susceptibilities. It is usually the absorption of near ultraviolet (UV) wavelengths which leads to bond-breaking reactions and the concomitant loss of useful physical properties and/or discoloration [Citation1].
Exposure to sunlight can have adverse effects on the useful great interest of plastic products. Ultraviolet (UV) radiation can break down the chemical bonds in a polymer. Photo-degradation causes cracking, chalking, color changes and the loss of physical properties [Citation2,Citation3].
Poly(vinyl chloride) is one of the most extensive thermoplastic materials in the world due to its valuable properties, wide applications, high chemical resistance, barrier properties and low cost [Citation4].
During processing, storage and utilization, PVC degrades as it is exposed to high temperatures, high mechanical stresses or ultraviolet light, all in the presence of oxygen. Degradation of the polymer occurs by successive elimination of hydrogen chloride (HCl), which is called dehydrochlorination, yielding long polyenes, which are consequently causing discoloration, deterioration of the mechanical properties and a lowering of the chemical resistance [Citation5].
There is a great interest at present in the photo-oxidative degradation of polymeric materials because macromolecules have increasingly widespread commercial applications [Citation6].
Almost all synthetic polymers require stabilization against the adverse effect, with the development of synthetic resins, it became necessary to look for ways and means to prevent or at least reduce the damage caused by the environmental parameters, light, air and heat. This can be achieved through addition of special chemicals, light stabilizers or UV stabilizers, that have to be adjusted to the nature of the resin and the specific application considered [Citation7–Citation9].
The photostabilization of polymers may be achieved in many ways. The following stabilizing systems have been developed, which depend on the action of stabilizer: (a) light screeners, (b) UV absorbers, (c) excited state quenchers, (d) peroxide decomposers and (e) free radical scavengers, of these it is generally believed that types (c–e) are the most effective [Citation10].
2 Poly(vinyl chloride)
Poly(vinyl chloride), better known by its abbreviation PVC, is one of the most versatile plastics [Citation11], . It is the second largest manufactured resin by volume worldwide [Citation12]. Poly(vinyl chloride) is second only to polyethylene among the five kinds of general plastic materials, which was widely used in industries including architecture, electronic, chemical engineering, packaging, transportation [Citation13–Citation15].
3 The origin of PVC and its subsequent development
The polymerization of vinyl chloride monomer (VCM) is known since 1872. Baumann was the first who produced poly(vinyl chloride) by accident. He exposed VCM to sunlight and obtained a white solid material that could be heated up to 130 °C without decomposition [Citation16]. Poly(vinyl chloride) has been produced commercially for 50 years. It was first produced in Germany and USA in the early 1930s (introduced in small quantities in different types of products), but its extensive use did not start until the 1939–1945 War when mixtures with certain organic liquids (plasticizers) producing a flexible material found wide application as a rubber substitute, particularly in those countries denied access to natural rubber supplies [Citation17]. The production and use of rigid PVC increased dramatically in the early sixties.
4 Stereo regularity of poly(vinyl chloride)
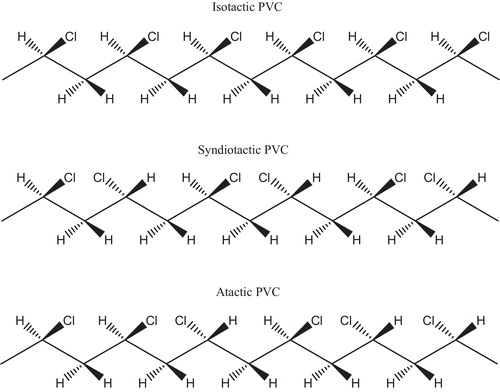
Stereo regularity (tacticity) refers to spatial isomerism in vinyl polymers and describes the arrangement of side groups around the asymmetric segment of vinyl-type repeat units, (–CH2–CHR–). Consequently, three different forms of polymer chain results in thermoplastics: atactic, isotactic and syndiotactic [Citation18]. shows the regular arrangement of the side group Cl in PVC polymer: in isotactic form all side groups on the same side of the polymer chain and in syndiotactic form side groups alternate regularly on either side of the chain. Atactic form describes the random attachment of the side groups about the back-bone chain.
5 Production of poly(vinyl chloride)
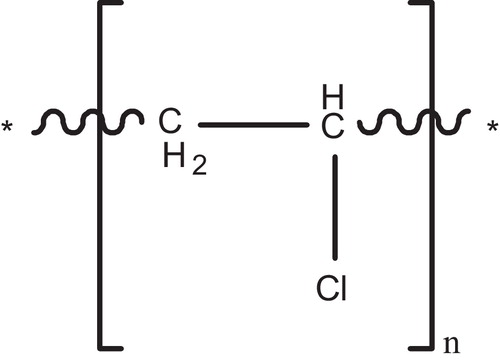
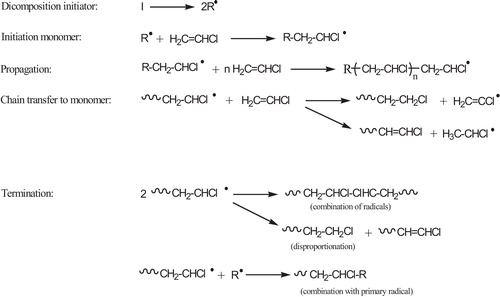
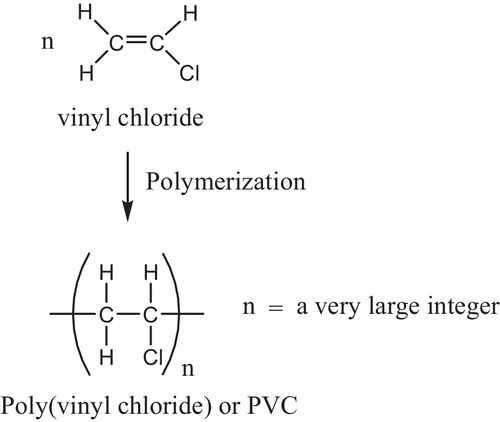
PVC is manufactured by three different processes: suspension, bulk or mass and emulsion polymerization. The suspension process, however, embraces 80% of all commercial productions of PVC [Citation19]. Polymerization of VCM occurs according to a free radical addition process, which includes initiation, propagation, chain transfer to monomer and bimolecular termination steps [Citation20], .
The overall reaction describing the PVC polymerization was shown in .
6 Uses of poly(vinyl chloride)
The low production costs and the great versatility of vinyl chloride polymers are the two major reasons for their large share on the plastic market [Citation21], which was widely used in industries including architecture, electronic, chemical engineering, packaging, transportation [Citation22]. The good performance of poly(vinyl chloride) products have increased the utilization of this polymer in building, mainly in exterior applications, such as window profiles, cladding structure and siding [Citation15]. The polymer can be converted into many different products exhibiting an extremely wide range of properties both physical and chemical by using modifying agents, such as plasticizers, fillers and stabilizers [Citation21]. The products can range from a flexible garden hose to a rigid drainpipe, from flexible sheets for raincoats to rigid sheets for packing, from soft toys to upholstery. Eventually, PVC compositions have succeeded in displacing materials such as rubber, metals, wood, leather, textiles, conventional paints and coatings, ceramics, glass.
In North America, PVC is mainly used for pipes and sidings, while in Europe and Asia, most use is for pipes and window frames. Builders in Japan have begun to use more PVC windows, in part because of their superior insulating properties to reduce heating and cooling costs. Demand is growing strongly in China for construction materials as well as consumer goods. Flexible PVC is used for film and sheet, wire and cable insulation, floor coverings, synthetic leather products, coatings and many other consumer goods.
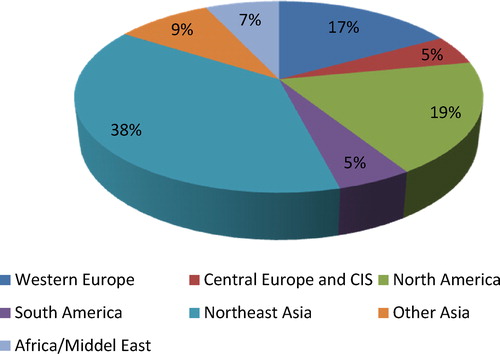
7 World consumption of poly(vinyl chloride)
PVC is a global product, manufactured by over 100 companies in approximately fifty countries. Practically all incremental capacity during 2005–2008 was installed in China, and to a lesser extent, India and the Middle East. Worldwide PVC consumption is represented by regions in .
Demand for flexible PVC has declined in the industrialized world, but continues to rise in certain countries such as China and India [Citation23]. The projected consumption of PVC in the near future (1998–2010) is much higher in the developing world and in countries in transition. Estimated demand for Asia alone is more than that for the USA, Canada and the European Community combined [Citation24]. However, strong Chinese demand has been accompanied by the rapidly expanding construction of local PVC production sites. In 2007, China already accounted for over 30% of the world’s PVC production capacity of approximately 41 million t. At a total demand of 7.8 million t of PVC in 2007 – and an average annual growth rate of 4.9% from 2004 through 2007 – Europe (incl. the CIS nations) consumes approximately 22% of the worldwide quantity of PVC and is thus the third largest regional market after America and Northeast Asia. The decisive force behind this has been strong demand in Central and Eastern Europe. Russia’s consumption alone grew by double-digits. But even in the mature Western European market, an average annual growth of 2.8% could be chalked up from 2004 through 2007.
8 Aging of poly(vinyl chloride)
Aging of poly(vinyl chloride) during processing and exploitation is the subject of numerous investigations. Usually, most investigations are devoted to the study of dehydrochlorination of a pure polymer in an inert atmosphere. PVC is most often used as a plasticate in practice (i.e., mixed with plasticizers), the amount of which may reach 70% of the total mass. All this makes the problem of PVC aging an extremely complex event and requires consideration of all processes proceeding under natural conditions of processing and exploitation [Citation25]. In the general case, the following processes can proceed during PVC-plasticate aging:
| • | PVC dehydrochlorination. | ||||
| • | Thermooxidative degradation of PVC and plasticizers. | ||||
| • | Cross-linking. | ||||
9 Main problem of poly(vinyl chloride)
The low cost and excellent performance of poly(vinyl chloride) make it a very attractive and suitable plastic for a wide variety of applications. However, PVC suffers from poor thermal and light stability. It undergoes rapid autocatalytic dehydrochlorination upon exposure to heat and light during its molding and use, respectively [Citation26,Citation27]. As a result, conjugated polyene sequences are formed from the beginning of the reaction, and they give rise to discoloration of the polymer and seriously change its physical properties [Citation28]. Degradation also causes a drastic change in the mechanical properties of the polymer, which is accompanied by a decrease or increase in its average molecular weight as a result of either chain scission or crosslinking of the polymer molecules, respectively [Citation29].
10 Thermal degradation of poly(vinyl chloride)
Poly(vinyl chloride) decomposes at a temperature lower than its processing temperature [Citation30]. The ideal structure of PVC is a linear structure formed by head to tail addition of monomer molecules to the growing polymer chain [Citation31]. Thermo gravimetric analysis on low molecular model compounds such as 2,4,6-trichloroheptane, 2-chloropropane and 2,4-dichloropentane, corresponding to the regular head-to-tail structure of PVC containing secondary chlorines only, shows that these model compounds are stable up to at least 200–300 °C. Commercially available PVC, on the other hand, would already degrade around 120 °C, if it were not stabilized before processing [Citation32–Citation34].
11 The causes for the low thermal stability of PVC
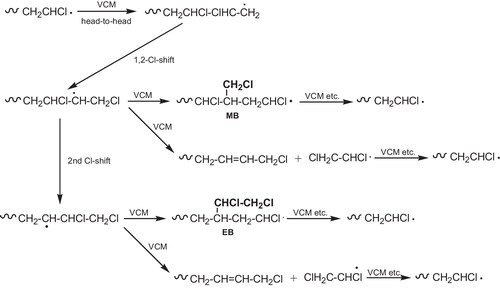
The main problem around PVC is its low thermal stability caused by the presence of defects in the molecular structure. Various defect sites in the polymer chain are thought to be responsible for this instability. Possible defect structures in PVC chains are allylic chlorine [Citation35,Citation36], tertiary hydrogen and chlorine atoms [Citation37,Citation38], end groups such as double bonds [Citation39–Citation41], oxygen-containing groups, peroxide residues [Citation42,Citation43], and head-to-head structures [Citation44]. In addition to these abnormalities, the steric order of the monomer units (tacticity) may have some influence on the degradation [Citation45,Citation46]. Some of them seem to affect the thermal stability while others are completely harmless. The frequently occurring branches and the most important types of branches concerning the thermal stability of PVC are described below:
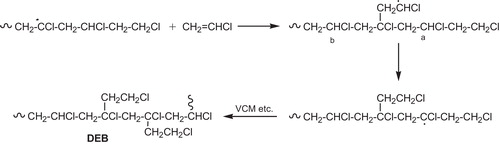
| • | Chloromethyl (MB) [Citation47–Citation49] and 1,2-dichloroethyl branches (EB) [Citation50], result from one or two successive 1,2-Cl shifts respectively, followed by regular chain growth as is shown in . | ||||
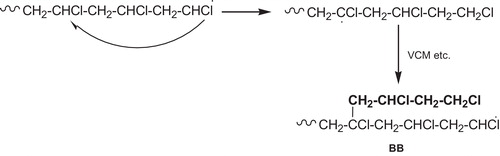
| • | The 2,4-dichloro-n-butyl branch (BB) is formed via a 1,5-backbiting mechanism [Citation51], . | ||||
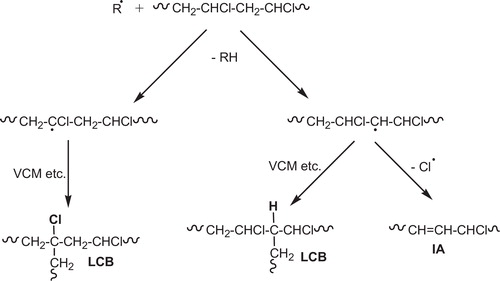
| • | Long chain branching (LCB), results from hydrogen abstraction, from a chloromethylene or a methylene unit of a polymer chain, by a growing macroradical or possibly a chlorine atom [Citation37,Citation52], . | ||||
| • | Diethyl branches (DEB) [Citation53,Citation54], seem also to be present in PVC fractions produced at very high VCM conversions and therefore when monomer supply is almost exhausted, . | ||||
| • | Oxygenated structures, . | ||||
12 Thermooxidative degradation of poly(vinyl chloride)
The following processes proceed during thermooxidative PVC degradation [Citation55]:
| • | Oxygen absorption. | ||||
| • | Hydrogen chloride extraction. | ||||
| • | Extraction of various volatile products. | ||||
| • | Decrease of mass and molecular mass of the polymer. | ||||
13 Chemical degradation of poly(vinyl chloride)
Many factors, such as temperature, humidity and solar radiation cause degradation in polymers [Citation56]. Plastic materials when exposed to sunlight slowly loose their physical and mechanical properties due to degradation [Citation57]. Poly(vinyl chloride) is a polymer which is very sensitive to the weathering action and this restricts its outdoor applications. This occurs mainly because of changes in mechanical properties and color [Citation58].
14 Photodegradation of poly(vinyl chloride)
There is a great interest at present in the photodegradation of polymeric systems, and this is reflected in the large number of research papers and other scientific publications that appear each year in this subject area. A major reason for this interest is that macromolecular materials have increasingly wide commercial applications where outdoor durability is an important consideration. In this context, all commercial organic polymers will degrade in air when exposed to sunlight, although there is a very wide range of photo-oxidative susceptibilities. It is usually the absorption of near ultraviolet (UV) wavelengths which leads to bond-breaking reactions and the concomitant loss of useful physical properties and/or discoloration [Citation1]. Under UV irradiation, and in the presence of oxygen and moisture, PVC undergoes a very fast dehydrochlorination and peroxidation process with the formation of polyenes [Citation58]. Degradation also causes a drastic change in the mechanical properties of the polymer, which is accompanied by a decrease or increase in its average molecular weight as a result of either chain scission or crosslinking of the polymer molecules, respectively [Citation42,Citation59–Citation61].
15 Photooxidative degradation of poly(vinyl chloride)
Long term exposure to sunlight leads to the degradation of plastic materials [Citation62]. UV energy absorbed by plastics can excite photons, which then create free radicals. While many pure plastics cannot absorb UV radiation, the presence of catalyst residues and other impurities will often act as free radical receptors, and degradation occurs. It only takes a very small amount of impurity for the degradation to occur. In the presence of oxygen, the free radicals form oxygen hydroperoxides that can break the double bonds of the backbone chain leading to a brittle structure. This process is often called photo-oxidation. However, in the absence of oxygen there will still be degradation due to the cross-linking process [Citation63]. Poly(vinyl chloride) has poor light stability in the wavelength range of 253–310 nm, presumably due to the presence of unsaturated (C=C) bonds, carbonyl, hydroperoxide, and hydroxyl groups in polymer chains. The relative activity of one or another chromophore in initiating PVC photodegradation is determined primarily by two factors: their ability to absorb UV light in the wavelength range under consideration and their participation in the formation of active particles (radicals) which cause degradation of the polymer chains. The alkene unsaturated (C=C) bonds (both internal and terminal) cannot be the primary initiators of PVC photodegradation under the action of sunlight with (λ > 250) nm because they absorb only the UV light at (λ < 200) nm. The absorption by conjugated (C=C) bonds (dienes, trienes, etc.) shifts toward the longer wavelengths [Citation64]. The color changes from white to yellow, brown, and finally to black while the properties of the material deteriorate [Citation65].
The exposure of vinyl chloride polymers to light at 250–350 nm develops typical signs of degradation in polymer samples [Citation55]:
| 1. | discoloration from natural to dark-brown or black, | ||||
| 2. | surface cracking, | ||||
| 3. | brittling or softening of the material, | ||||
| 4. | variation of the mechanical properties (tensile strength, ultimate elongation, impact strength, and elasticity modulus), | ||||
| 5. | change of transparency, | ||||
| 6. | formation of a deposit on the material surface. | ||||
Degradation of PVC due to weathering is a free-radical mechanism started by the absorption of sufficient energy to break chemical bonds. Weak sites susceptible to degradation, as well as mechanisms of yellowing, oxidation, bleaching and surface erosion, have been investigated and described in several papers [Citation66,Citation67].
The photo-oxidation of PVC can be described by the following sequence [Citation68,Citation69]:
| 1. | Photolytic formation of polyenic sequences with growing conjugation lengths by multistep photo-chemical excitations. This photolysis can be initiated by excitation of chromophoric defects with the structure of α-chlorinated dienes. These reactions lead to a marked discoloration and the so-formed polyenic sequences are readily photo-oxidized in the presence of molecular oxygen; photobleaching is then observed to occur. | ||||
| 2. | Photochemical oxidation that can be initiated by Cl* formed along with the polyenic sequences and that leads to the formation of the following main products: α,α′-dichloroketones, β-chloro-carboxylic acid, and acid chlorides. | ||||
| 3. | Cross-linking of PVC by recombination of the in-chain macroradicals. | ||||
In the presence of the UV radiation, oxygen seems to attack the PVC chains randomly or on sites that are not involved in the mechanism of thermal degradation [Citation40].
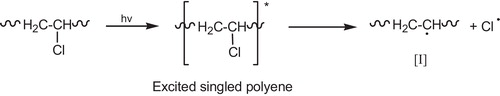
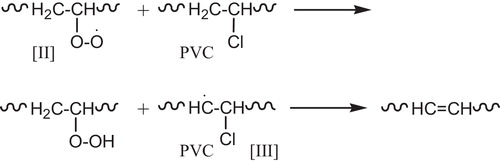
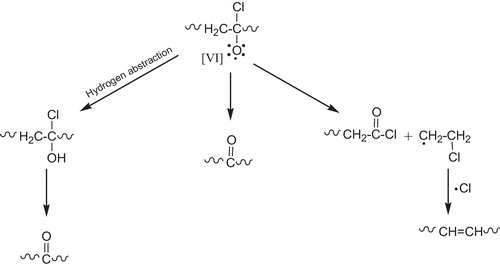
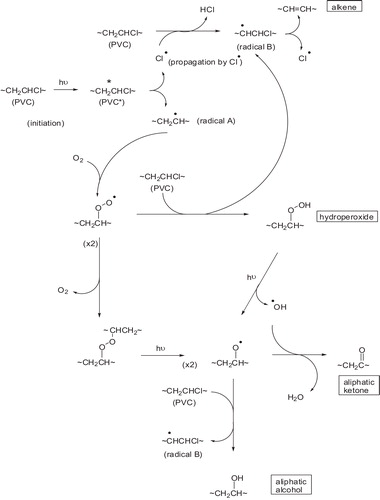
When PVC is photolyzed in the presence of (O2), the primary photochemical processes occur (the excited singlet polyenes). Those excited states will thus disappear by the different routes including the allylic (C–Cl) bond cleavage with formation of (Cl–) radical and polyene radical [I], .
The polyenyl radical [I] which bears no (Cl) atom in the α-position is very likely to be scavenged by (O2) to give peroxy radical [II], see .
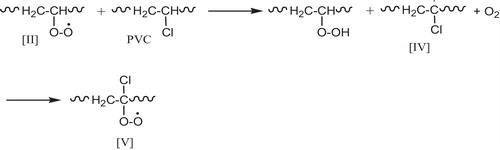
Rate constant measurement on related model compounds suggest that peroxy radical [II] react with the (–CH2–) and (CH–Cl) groups on PVC at rates that are comparable. The attack on (CH2) group yields radical [III], .
This radical contributes to the chain-dehydrochlorination of PVC. The attack of II on the (–CH–Cl) group yield radical [IV] which possesses no labile β-chlorine and is likely to react with (O2) to give a γ-chloroalkyl peroxy radical [V], see .
The main oxidation products of PVC are expected to result primary from the various reactions of this radical [V], there are two major routes of the fate (disappearances) of the γ-chloroalkyl peroxy radical. The hydrogen abstraction from PVC with the formation of hydroperoxide groups, .
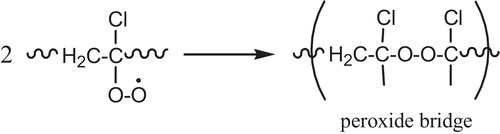
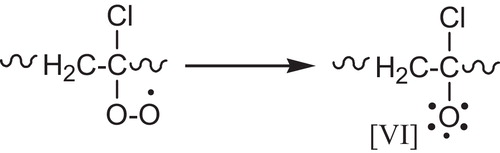
The bimolecular interaction, the later leads to the formation of either a peroxide bridge (termination reaction), see , the formation of alkoxy radicals [VI], .
The most usual reaction of alkoxy radicals [VI] is the hydrogen abstraction. The unstable α-chloroalcohol formed by reaction of alkoxy radical with PVC will rapidly decompose into the corresponding ketone, .
Tertiary alkoxy radicals are also proved to be stabilized by β-scission which may involve either (C–Cl) bond cleavage to form ketone or/and (C–C) bond cleavage to form polyene [Citation70], see .
The overall mechanism now suggested for the photooxidation of PVC to account for the main primary products can be summarized by the reaction shown in , [Citation71] where P– represents radical ∼(CH=CH)n·CHCH2∼ or ∼CH2·CClCH2∼. The two major chain process which develop simultaneously are clearly apparent [Citation72].
16 Dehydrochlorination of poly(vinyl chloride)
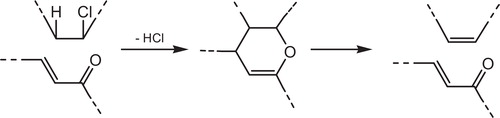
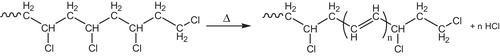
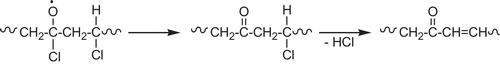
The dehydrochlorination most likely proceeds by a chain mechanism involving radical intermediates [Citation27]. The presence of hydrochloride, the reaction product, and oxygen in the surrounding accelerates dehydrochlorination. That is why it is worthwhile, first, to consider the reaction of noncatalytic dehydrochlorination. During processing, storage and utilization, PVC degrades as it is exposed to high temperatures, high mechanical stresses or UV light. Degradation of the polymer occurs by successive elimination of hydrogen chloride (HCl), which is called dehydrochlorination [Citation73], see . The reaction of dehydrochlorination proceeds in PVC at a noticeable rate in a high-temperature range 150 °C [Citation5].
17 Oxygen effect on dehydrochlorination
Dehydrochlorination is significantly accelerated in the presence of oxygen [Citation74]. This is connected to the formation of oxygen-containing groups, which initiate dehydrochlorination, see .
18 Protection against environmental degradation in polymers
Polymeric materials, synthetic, semisynthetic and natural are photodegradable when exposed to the environment [Citation75–Citation77], because of the fact that polymers are not pure compounds. For example, they commonly contain additives (e.g., stabilizers, pigments, fillers, finishing agents, etc.) and impurities (e.g., oxidation products, catalyst residues, etc.) which may very well influence or even dominate the photochemistry of the polymeric system [Citation1].
Plastics are commonly protected against such deterioration by the addition of antioxidants, light and heat stabilizers [Citation78]. Ultraviolet light stabilizers are used widely in plastics, cosmetics, and films. The main purpose of UV stabilizer is to prevent polymers from photodegradation or photocrosslinking caused by ultraviolet light presented in sunlight and artificial light source [Citation79].
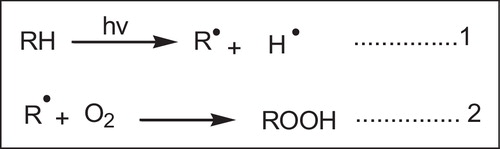
Physical or chemical stabilization against environmental degradation in polymers may be achieved by blocking any of the steps in the deterioration process. Since the effects of UV radiation are the most serious threat to environmental durability, a number of techniques have been developed to counter them. UV radiation may be excluded by various coatings or paints. Fillers such as carbon black may also be used as screening agents, or the radiation may be absorbed harmlessly by chemical agents which dissipate the photon energy without chemical change. Radical scavengers may be employed to terminate chain radicals and halt the propagation steps, and various deactivators are available that serve to stabilize the hydroperoxide groups formed during photolytic oxidation (see Eqs. (1) and (2)):
The photostabilization of polymers may be achieved in many ways. The following stabilizing systems have been developed, which depend on the action of stabilizer: light screeners, UV absorbers, excited state quenchers, peroxide decomposers and free radical scavengers, of these it is generally believed that excited state quenchers, peroxide decomposers and free radical scavengers are the most effective [Citation6]. Most or, indeed all stabilizers are believed to be multifunctional in their mode operation. This view is complicated by the fact that the mechanism involved in photo-oxidation and these, in turn depend on the polymer structure and other variables, such as manufacturing, operation, processing, conditions [Citation80].
19 How to avoid UV degradation
There are several ways of avoiding UV degradation in plastics by using stabilizers, absorbers or blockers. For many outdoor applications, the simple addition of carbon black at around a 2% level, will provide the protection for the structure by the blocking process. Other pigments such as titanium dioxide can also be effective. Organic compounds such as benzophenones and benzotriazoles are typical absorbers which selectively absorb the UV and re-emit at a less harmful wavelength, mainly as heat. The benzotriazole type is good, as it has a low color and can be used at low dose rates below 0.5%. The other main mechanism for protection is to add a stabilizer, the most common being a HALS (hindered amine light stabilizer). These absorb the excited groups and prevent the chemical reaction of the radicals. In practice, the various types of additives used are in combinations, or are compounded into the original polymer to be produced as a special grade for UV protection. It may be attractive to add antioxidants to some plastics to avoid photo-oxidation, but care must be taken that the antioxidant chosen does not act as an UV absorbent, which will actually enhance the degradation process [Citation81].
20 Additives, reinforcements and fillers
Additives: are ingredients added to the polymer to stabilize, modify or enhance its performance.
Stabilizers: are used to maintain the polymer’s strength, flexibility and toughness; in other words, the attributes of the polymer’s original molecular architecture.
Modifiers: improve/alter the polymer’s performance; e.g., Slip Agents, Antistats, Antiblocks, Processing Aids, Fillers.
Fillers: such as SiO2, CaCO3, Talc, or TiO2 are used to improve physical properties, or dilute the matrix with something less expensive than the polymer itself.
These substances change properties of polymers and render them more adaptable and versatile. Polymers make excellent matrices for reinforcing fibers (the resultant composites are known as polymer–matrix-composites, PMC) and excellent binders for pigments such as TiO2 in paints.
Most additives fall into one of the following categories:
| • | Modifiers: such as plasticizers, nucleating agents, clarifiers, impact modifiers, (e.g., rubber particles) blowing agents, colorants and coupling agents. | ||||
| • | Stabilizers: including antioxidants, heat and UV stabilizers, fire retardants, antistatic agents and fungicides. | ||||
| • | Processing aids: lubricants, compatibilizers (e.g., Struktol in wood-plastic composites) reducers of melt temperature/pressure, etc. | ||||
Care should be exercised in the usage of additives: even the most useful additive can have detrimental effects, for example, carbon black greatly reduces the tracking resistance of a material and should be avoided in electrical applications [Citation82]. Combining several types of additives into synergistic “packages” is becoming popular, also formulation of liquid systems, rather than powder, for ease of mixing. The choice of additives must also be dictated by health and safety considerations. Much work is underway to reformulate PVC additives (incidentally the largest % of additives are used in PVC) to eliminate heavy metals such as lead, barium and cadmium.
21 Factors governing choice of stabilizer
It is very important that the UV stabilizer (or combination of UV stabilization) should be selected to suit the exact application. It is therefore important to identify where an end use product will be used and the required durability. Other factors that affect the choice of UV stabilization package include product dimensions, type and color of pigments present and application information such as food contact.
22 UV light absorbers
A UV light absorber is a type of light stabilizer which functions by competitive absorption of the UV light energy, causing the photodegradation of a polymer formulation. The UV absorber competes with all chromophores in the formulation and dissipates the absorbed energy as heat. The chromophores are UV light absorbing chemical groups in the polymer formulation. These may include the polymer itself and other additives, such as halogenated flame retardants, fillers, and pigments. In effect, the UV light absorber inhibits the first step in the polymer photodegradation process, which is photoinitiation. The UV light absorber can be used in combination with other types of light stabilizers which are designed to inhibit the other, subsequent steps in the photodegradation processes. Also, UV light absorbers may function as UV screeners in plastics, coatings, and cosmetic sunscreens to reduce or eliminate the transmission of UV light energy, protecting UV-sensitive substrates.
The factors involved in the effectiveness of the competitive UV light absorption stabilization mechanism are as follows:
| (1) | The UV wavelength region causing photodegradation of the polymer formulation. | ||||
| (2) | UV absorptivity of the polymer formulation in this region. | ||||
| (3) | UV absorptivity of the UV absorber in this region. | ||||
| (4) | Thickness of the polymer. | ||||
| (5) | Concentration and dispersion of the UV absorber. | ||||
| (6) | Inherent light stability of the UV absorber. | ||||
| (7) | Permanence of the UV absorber. | ||||
| (8) | Chemical reactivity of the UV absorber in the photodegradation process. | ||||
| (9) | The UV light and thermal exposure conditions of the polymer formulation. | ||||
The optimum amount of UV absorber varies with each chemical type and application, but in most cases is in the 0.25–1.0% concentration range. Other factors to consider while selecting the proper type and amount of UV absorber are color contribution, FDA sanction for food packaging applications, and, of course, add-on cost. The UV absorbers are incorporated into plastic substrates by dry blending, extrusion compounding, and then conversion into the final product by various processes such as injection molding, blown film extrusion, multifilament spinning, and sheet extrusion. Also, UV absorber dispersions can be prepared and dyed onto textile fabrics, which will not only UV-stabilize the polymer substrate but also improve the color stability of the dyes used to color the fabric. The use of UV absorbers as UV screeners is practiced in a multitude of applications, such as sunglasses, interocular lenses, auto windshield films, solar control glazing films, photographic products, greenhouse films, product packaging, and cosmetic sunscreens. In each application, the polymer type, the UV absorber type, the thickness of the plastic product, and the concentration of the UV absorber need to be considered to meet the reduced UV percentage transmission requirements of the application [Citation83].
23 UV stabilization mechanisms and strategies
The initial emphasis in light stabilizer development was the inhibition of the photoinitiation processes. This UV stabilization strategy consisted of the incorporation of additives into the polymer or coating formulation, which either absorbed UV light energy or acted as quenchers of the photoexcited states of the chromophores. In the former case the UV absorber competes with the chromophores for the harmful UV light energy, which is preferentially absorbed by the UV absorber and dissipated harmlessly as heat [Citation1]. In this competitive absorption mechanism, the UV absorber must have relatively high absorptivity at the wavelengths of UV light to which the polymer is photosensitive (activation spectra maxima). In the latter case, the quencher returns the excited states to ground states by energy transfer processes. In both cases, radical formation is inhibited and the outdoor service life of the polymer is extended, depending on the efficiency of these stabilization processes.
Also, light stabilizers have been developed to inhibit the subsequent oxidative processes by radical scavenging and hydroperoxide decomposition mechanisms. In these processes, the light stabilizer molecules undergo chemical reactions with the propagating intermediates in the polymer photo-oxidation processes. In some cases the light stabilizer is gradually consumed and in others the stabilizing chemical groups are regenerated to some degree in the stabilization process. This latter process has been used to explain the effectiveness of HALS [Citation84–Citation86].
The third strategy is to combine a UV absorber with a radical scavenger or a hydroperoxide decomposer to provide more efficient and sometimes synergistic UV-stabilizing activity. Also, effective combinations of radical scavengers and hydroperoxide decomposers have been reported [Citation87]. And finally, as part of a total system strategy, melt-processing antioxidants can be added to the polymer formulation to minimize the formation of UV-absorbing chromophores from thermal oxidation during thermal processing.
Light stabilizers need to satisfy a number of technical, as well as commercial, requirements. Technical requirements include:
| (1) | strong, broadband UV absorption (for UV absorbers), | ||||
| (2) | high inherent stability to UV light energy, | ||||
| (3) | good solubility–migration balance in the polymer substrate, | ||||
| (4) | low volatility and extractability, | ||||
| (5) | low color and odor, | ||||
| (6) | minimum adverse interactions with other system components, and | ||||
| (7) | good storage stability. | ||||
In addition to the primary light stabilization functional group, substituents are used to fine-tune performance as follows:
| 1. | To vary the molecular weight to change the volatility, solubility–migration balance, and extractability. | ||||
| 2. | To vary the chemical reactivity of the functional group. | ||||
| 3. | To enhance the UV absorption or the inherent light stability. | ||||
| 4. | To add a reactive group to achieve permanence. | ||||
24 Light stabilizers for plastic materials
To provide an appropriate protection against UV radiation, several stabilizing systems can be utilized in plastic materials. The most important types of light stabilizers are ultraviolet light absorbers, energy transfer agents or quenchers, as well as hindered amine light stabilizers. A brief description of these different light stabilizers is given below.
24.1 UV light absorbers
Absorbers convert harmful ultraviolet radiation to harmless infrared radiation or thermal energy, which is dissipated through the polymer matrix. They can be either transparent as hydroxybenzophenone or opaque like carbon black.
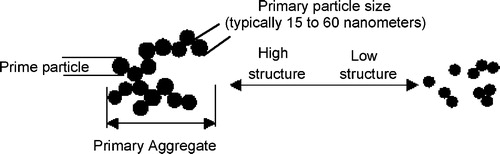
24.1.1 Carbon black
The most common type of UV protection for polymeric products is carbon black. Carbon black is a particulate form of industrial carbon; it consists of very fine particles (the prime particles) fused together to form the primary aggregates. The microstructure of the carbon black is illustrated in .
The UV absorbing efficiency of the carbon black is governed by the average prime particle size and structure. Primary aggregates composed of finer prime particles present greater surface area for optical absorption than the primary aggregates composed of larger prime particles. Thus, UV absorption increases as prime particle size decreases. However, with prime particles below 20 nm the UV stabilizing efficiency tends to level off as light scattering becomes more significant with further decrease in particle size [Citation88]. The carbon black particle size used for UV protection of polymers used for geosynthetics is typically in the range of 22–25 nm. Typical applications for carbon black as a UV stabilizer in plastics are exterior pipe, polyolefin agricultural film, pond linings, automotive parts and exterior cable jacketing (PVC, PE, etc.).
24.1.2 Titanium dioxide
For non-black polymeric materials, UV screeners are added to protect the polymer from UV degradation. Similar to the carbon black, UV screeners work by absorbing UV light, dissipating the energy harmlessly as heat. The most widely used non-black UV screener is TiO2. Turton and White [Citation89] showed that the addition of 1% TiO2 can significantly reduce the degradation of PP using a UV-fluorescent condensation device. Furthermore, they found the UV degradation of PP to be lower in samples with TiO2 than HALS stabilizers. Another group of UV screeners are non-colored.
24.1.3 Hydroxybenzophenone and hydroxyl phenyl benzotriazole
These well-known UV absorber types offer the advantage of being suitable for natural or transparent applications. To provide a good protection to the plastic material, a certain absorption depth is needed (part thickness) which makes these absorbers inefficient in thin items such as films (below 100 μm), fibers or tapes [Citation90].
24.2 Excited state quenchers
One approach to imparting long-term outdoor stability to polymeric systems is to add to them small amounts of compounds which will quench the electronic excitation energy associated with specific chromophores as a result of photon absorption. Carbonyl groups, hydroperoxides and singlet molecular oxygen (or its precursors) are chromophores commonly believed to be involved in the photodegradative mechanisms for numerous hydrocarbon polymers [Citation1].
This type of light stabilizer functions by bringing ‘excited’ state polymer molecules (chromophores) back to their stable state, preventing bond cleavage and finally formation of free radicals. If the energy could be transferred to a molecule and dissipated harmlessly, radicals would not be formed. The nickel chelates have been reported to act primarily as excited state quenchers [Citation91].
Singlet oxygen can be quenched with certain additives but there is no evidence that it is a major contribution to the photooxidation of any polymer. Efficient photostabilization of hydrocarbon polymers really requires peroxide decomposition and radical scavenging [Citation1].Most nickel UV stabilizers also have been found to provide hydroperoxide decomposing or radical scavenging activity. The nickel chelates usually add tan or green color to the polymer system. They perform better than UV absorbers in thin cross sections and in highly pigmented systems.
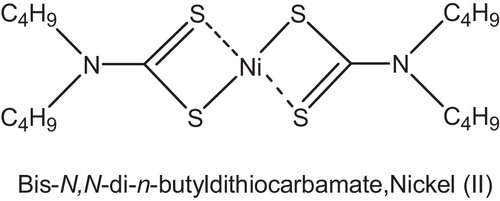
Nickel di-butyldithiocarbamate (Rylex NBC), , was introduced in the late 1950s. It had many drawbacks, such as high color contribution and volatility, but was a potent light stabilizer. It functioned mainly through hydroperoxide decomposition.
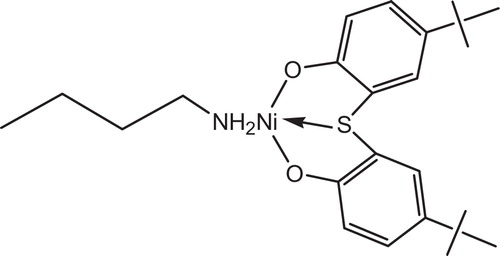
[2,2-Thiobis(4-octylphenolato)]-n-butylamine nickel, see , (CYASORB® UV-1084 Light Stabilizer) was introduced in the early 1960s. This product continues to be used extensively in agricultural film applications, where its main advantage is resistance to deactivation by pesticides and soil fumigants, while providing good light stability. It is used alone in white on black mulch film products. Also, it is used in combination with 2-(2-hydroxy-4-octyloxyphenyl)benzophenone (CYASORB® UV- 531 light absorber) in greenhouse film products usually at a 2:1 ratio.
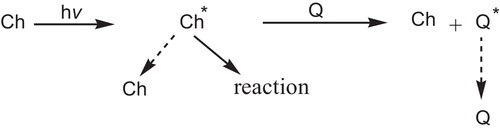
The excited state of a chromophore (Ch), , may revert to the ground state by some photophysical process(es) or it may react but it can also be made to transfer its excess electronic energy to a quenching entity (Q).
If energy transfer to the quencher can compete with reaction, decomposition etc. by Ch, and if Q can dissipate the excess energy harmlessly, then the system is stabilized. Energy transfer can occur efficiently only if the energy level of the quencher is below that of the chromophore [Citation91].
Quenching of electronically excited states (singlets or triplets) is frequently described in terms of two distinct phenomena:
| • | Long-range energy transfer, e.g., a dipole/dipole interaction as described by Forster is considered to operate between the chromophore and quencher even at distances >50 Å if there is significant overlap between the emission spectrum of Ch* and the absorption spectrum of Q. [Citation91] It has been suggested [Citation92] that this process is normally observed in the quenching of excited singlet states. | ||||
| • | Contact (collisional) energy transfer of various kinds is said [Citation93,Citation94] to require that Ch* and Q be within 10–15 Å. | ||||
In practice, the quenching of excited triplets is usually ascribed to collisional transfer. A number of questionable assumptions are made about the stabilization of polymers by additive quencher during outdoor exposure. For example, it is a mistake to assume that polymer stability will invariably be enhanced by quenching a particular chromophore unless there is unambiguous mechanistic evidence about the importance of that chromophore in key photoprocesses. Furthermore, it is frequently assumed that additives in macromolecular systems will be evenly distributed, and this would seem to be very unlikely.
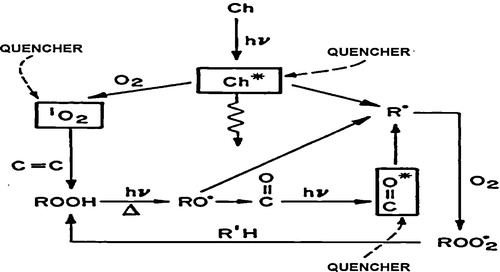
illustrates the types of reactions which occur in the photodegradation of hydrocarbon polymers. The potential applicability of quenching stabilizers is also indicated.
24.3 Hindered amine light stabilizers (HALS)
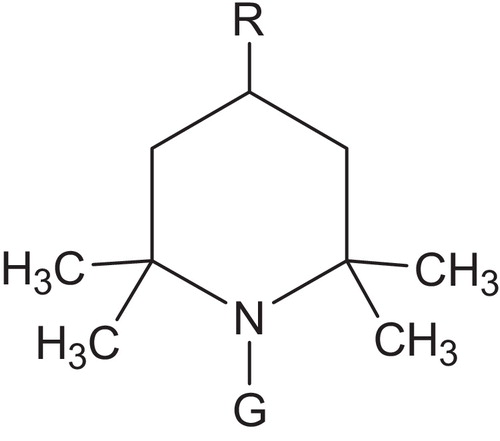
Hindered amine light stabilizers (HALS) have been well recognized for more than 25 years to be the most proficient UV stabilizers for a large number of polymers. This is particularly true for the protection of polyolefins against the degradative effects of light energy. The protective effects of HALS has enabled the growing use of polypropylene (PP) in the automotive industry. The main protective effect is the strong improvement in light stability achieved with the use of HALS. These stabilizers also impart thermal stability to plastics at temperatures below 130 °C. Although there are wide structural variations in commercial HALS products, all share the 2,2,6,6-tetramethylpiperidine ring structure, .
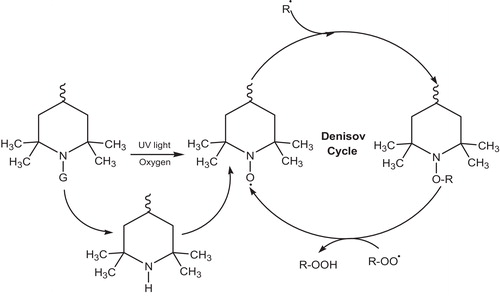
The nitroxide radical of this cyclic amine is the actual active light stabilizer. The nitroxide radical is generated in situ by photo-oxidation and photohydrolysis, depending on the substituent on the nitrogen atom [Citation95–Citation97]. The kinetics of nitroxide generation and consumption in coating matrices has been studied by electron spin resonance (ESR). Nitroxide consumption in coatings has been used to measure photoinitiation rates for photo-oxidation. In a sequence of reactions known as the Denisov Cycle, the nitroxide radical combines with other radical species in the coating matrix to form amino ether intermediates, which further react with radical species to regenerate the nitroxyl radical and form non radical chemical by-products. Thus the piperidinyl nitroxide radical may be thought of as a catalytic radical scavenger [Citation98,Citation99]. The Denisov Cycle is shown in .
Concurrently, the influence of structural, chemical, and physicochemical characteristics of HALS has been understood and improvements in their performances made. These advancements have led to several generations of HALS intended to cover a wide variety of applications. With these improvements, the confidence in the correlation between accelerated and outdoor weathering has grown, thus reducing the time necessary for evaluation and approval of new stabilizing products and systems. The consequence of this continuous growth in knowledge has been the appearance on the market of several generations of HALS, which can be historically summarized as follows: first generation-monomeric HALS, second generation-polymeric HALS, third generation-noninteractive HALS and fourth generation-synergistic UV absorber-HALS combinations [Citation100–Citation102].
2,2,6,6-Tetramethyl-4-oxy-piperidine N-oxyl (also known as N-Oxyl TAM), , was synthesized [Citation103], and found to be effective as a light stabilizer in plastics [Citation104]. However, the molecule had a low melting point, had limited thermal stability, imparted color to substrates, and was highly migratory. Further improvements were needed. Subsequent studies led to the discovery that the amine analogs of the N-oxyl compounds were highly active as stabilizers, with fewer undesirable side reactions [Citation105].
25 Other light stabilizer types
In addition to UV absorbers, excited state quenchers and HALS, other types of UV stabilizers which inhibit degradation processes subsequent to photoinitiation are used. These include hydroperoxide decomposers, radical scavengers and pigments.
25.1 Hydroperoxide decomposers
Hydroperoxides were found to be a key UV absorbing chromophore and an intermediate in the thermal and photooxidative mechanisms of many polymers. Reducing the hydroperoxide to a stable alcohol before it was thermolized or photolized into radical fragments was recognized to be very beneficial to polymer stabilization. Phosphites, nickel dithiocarbamates, cobalt dithiophosphinates, amidothiophosphates, and nickel thiobisphenolates, (CYASORB® UV-1084 Light Stabilizer) , all are capable of decomposing hydroperoxides, and provide a significant amount of light stabilizing activity.
Since hydroperoxides are decomposed by heat and light to generate radicals which feed the degradation processes, it is essential that they be decomposed into nonradical products. Phosphites, such as di-stearyl pentaerythritol diphosphate (Weston 618), see , and sulfur compounds, such as nickel dibutyl dithiocarbamate (Rylex NBC), provide this function and both provide UV-stabilizing activity in polyolefin formulations. Phosphites usually are used in combination with other UV stabilizer types.
25.2 Radical scavengers
Free radicals play a central role in a variety of chemical processes. The lack of methods with which to detect and identify very low concentrations of free radicals in condensed phases presents a major obstacle to understanding the impact of these highly reactive species on chemical and biological processes. Direct detection of radicals by electron paramagnetic resonance (EPR) or optical spectroscopies is generally not possible because of high reactivity or low steady-state levels of these species. More often, radicals are detected indirectly by employing radical traps or scavengers which react rapidly with transient radicals to form more stable products [Citation106,Citation107].
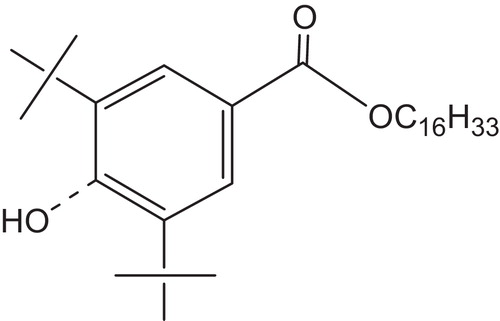
Reacting with the radicals generated from the excited states before they would react with oxygen or reacting with the peroxide radicals before they would abstract a hydrogen from the polymer would certainly be beneficial to the stabilization process. Hindered phenolic antioxidant radical scavengers developed for thermal stabilization of polymers were found to offer very little light-stabilizing activity. However, the unique hindered benzoate class of radical scavengers was soon discovered. The aliphatic benzoate n-hexadecyl 3,5-di-tert-butyl-4-hydroxybenzoate (CYASORB® UV-2908 Light Stabilizer), , does not photorearrange [Citation108] to a benzophenone UV absorber, but does offer superior light stabilizing activity, alone or in combination with other classes of stabilizers [Citation109,Citation110].
Although HALS are recognized as one of the greatest advances in light stabilizer technology, their basicity and reactivity limits their use in many applications. Some examples are acid-catalyzed, cross-linked, automotive coatings. Aliphatic benzoates can be substituted as radical scavengers in such applications where HALS cannot be used [Citation111].
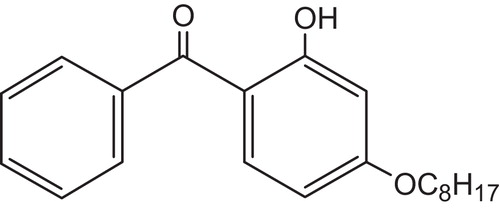
The UV stabilization of blue, V-2 flame-retardant stadium seating was approached on the basis of the pigment and FR type. A benzophenone, 2-hydroxy-4-n-octyloxybenzophenone (CYASORB® UV-531 light absorber), , was used at 0.5% to keep the blue pigment from fading and the aromatic bromine portions of the FR stable against UV radiation.
25.3 Pigments and fillers
Other factors such as product cross-section and opacity affect UV stabilizer effectiveness, depending upon the chemical type and mechanism of action. In fact, carbon black and many white pigments, such as rutile titanium dioxide and zinc oxide, at sufficient concentration levels can limit the penetration of UV light energy, providing high levels of UV stabilization. In other situations, unfavorable interactions between pigments, fillers, and UV stabilizers result in reduced light stability.
The influence of pigments in polymer photostability is not completely understood. If an absorbing pigment is introduced into a polymer, it acts as an inner screen for photo products. If these products are not photooxidized, they accumulate in the polymer matrix. Since pigments act as highly absorbing additives, photooxidative phenomena will be limited mainly to the surface of samples [Citation112,Citation113].
25.4 Antioxidants
Antioxidants are added to the polymer to prevent free radical chain reactions, particularly in the polyolefin products [Citation114].
Antioxidants are generally classified into two groups, according to their protection mechanism:
| • | Kinetic chain-breaking antioxidants (chain terminators, chain scavengers). They have capability to scavenge some or even all available low-molecular radicals (R–, RO–, ROO–, HO–, etc.) and polymeric radicals (P–, PO–, POO–) by a process called a chain-breaking electron donor mechanism. | ||||
| • | Peroxide decomposers, which decompose hydroperoxy groups (HOO–) present in a polymer. | ||||
Based on the previous research results [Citation115], the service lifetime of polyolefin geomembranes is initially governed by the consumption of antioxidants. The rate of antioxidant depletion is significantly faster under photo-oxidation than under thermooxidation. In recent years, the most common type of antioxidant for light stabilization is hindered amine light stabilizers (HALS), which is often called the light stabilizers. The UV degradation of unstabilized and HALS-stabilized PE and PP has been studied by many researchers [Citation116,Citation117].
For PVC, antioxidants are added to suppress the zip-elimination and oxidation reactions [Citation118,Citation119]. Regarding PET and PA, antioxidants are not commonly incorporated into the formulation for geosynthetic products since they are less susceptible to oxidation.
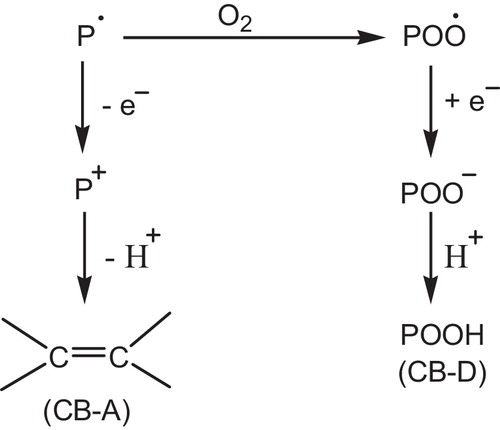
shows the two major anti-oxidant mechanisms. The chain breaking donor (CB-D) and chain breaking acceptor (CB-A). Also an antioxidant can act by preventive inhibition processes [Citation24,Citation120].
26 Stabilization of poly(vinyl chloride)
It is well known that all commonly used plastics degrade under the influence of sunlight and that is why the photostability of polymers is one of their most important properties. As a possible way to solve the problem of polymer stabilization, a number of different stabilizers have been successfully used [Citation29,Citation121].
Organic UV-stabilizers, generally with small molecular weight, include fluorescent compounds, phenyl-ester of benzoic acid, hydroxylbenzophenone, benzotriazoles, etc. In the addition of these stabilizers to plastic materials, problems such as migration, incompatibility, volatility, and solvent extraction will inevitably occur. It leads to a strong diminution of the materials utilization. To resolve such problems, many approaches have been developed, such as preparing reactive UV stabilizer [Citation6,Citation26], introducing compatible side chains, or chemically anchoring of the additive to the polymer backbone, etc. [Citation15]. Among these methods, preparing high molecular weight UV stabilizer is a highlight because, for most of the polymer materials, blending is the first choice to enhance their UV-resistance. Meanwhile, different high molecular weight UV-stabilizers can be prepared by the copolymerization of a reactive UV-stabilizer with other monomers.
In outdoor applications where the materials are exposed to UV solar radiation, the energy of this radiation is sufficient to initiate photochemical reaction leading to degradation.
Ultimate user acceptance of the PVC products for outdoor building applications will depend on their ability to resist photodegradation over long periods of sunlight exposure. To ensure weatherability, the PVC resin needs to be compounded and processed properly using suitable additives, leading to a complex material whose behavior and properties are quite different from the PVC resin by itself [Citation122].
A number of heterocyclic compounds including polydentate amines, crown ethers, bipyridines, naphthyridines, 2-aminobenzothiazol and 2-mercaptobenzothiazole have been bound with mainly polystyrene divinylbenzene copolymers or linked with poly(vinyl chloride) [Citation70,Citation123].
Recently, scientists have used substituted benzothiazole and benzimidozole ring [Citation70] as photostabilizers for rigid PVC. They have also used 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives as novel photostabilizers for rigid PVC [Citation124] and some synthesized organic compounds as N-substituted maleimides, N-phenyl pyrazolone, phenylurea, some glucoside derivatives, and other organic compounds to be used as photostabilizers for PVC [Citation125–Citation128].
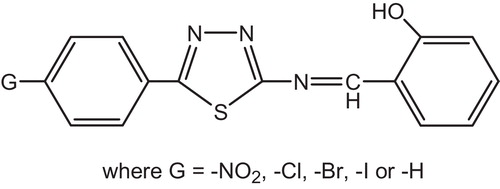
Yousif et al. have Investigated the photostabilization of PVC films by Schiff bases compounds containing four 1,3,4-thiadiazole rings [Citation29]. The structure of these additives are shown in .
Schiff base stabilize PVC by different mechanisms such as UV absorber, screener or by radical scavenger. These stabilizers provide very good long-term stability and are usually referred to these mechanisms.
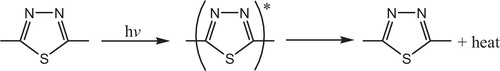
The rings of 1,3,4-thiadiazole play a role in the mechanism of the stabilizer process by acting as UV absorber [Citation124]. The UV light absorption by these additives containing 1,3,4-thiadiazole dissipates the UV energy to harmless heat energy, .
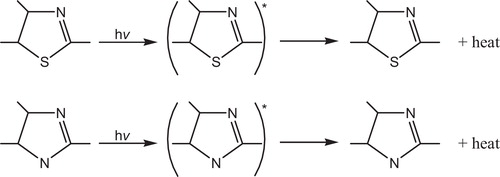
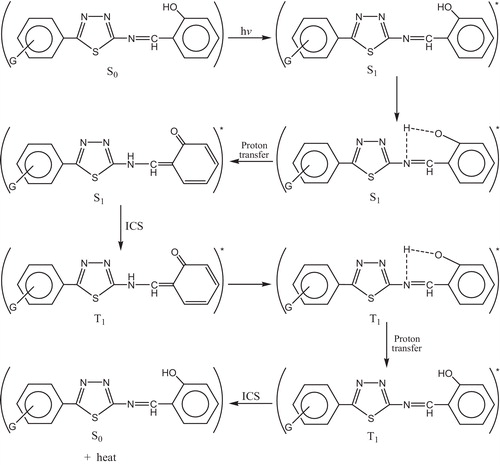
The most probable mechanism involved in a photostabilization is the change energy of absorbed photon to the intramolecular proton transfer. This reaction may occur by two proposed cycles, and . The first passes by intersystem crossing (ICS) process to the excited triplet state, while the second is referred to internal conversion (IC) process to the ground state.
Fig. 36 Mechanism of photostabilization of PVC through absorption of UV light and dissipation light energy as heat (IC).
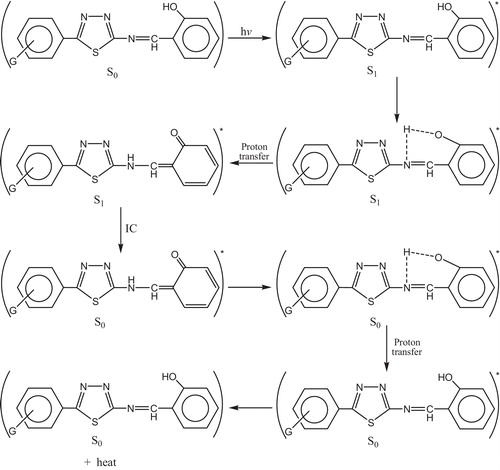
Fig. 37 Mechanism of photostabilization of PVC through absorption of UV light and dissipation light energy as heat (ICS).
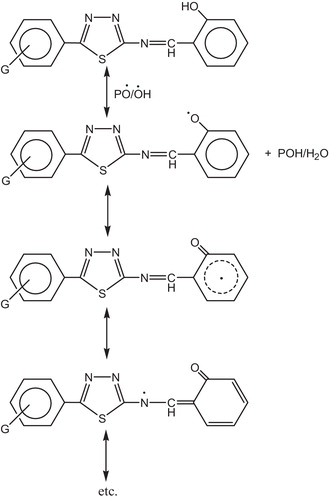
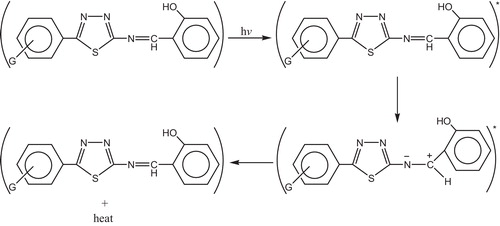
The hydroxyl group of the additive might act as radical scavenger for photostabilization process. Therefore this Schiff bases, besides acting as UV absorber they may also act as radical scavenger additives, .
Other mechanism explains the use of this compound as photostabilizer is by charge separated species which could be a form of the excited state such a structure would allow dissipation of energy through rotation on increased vibration about the central bond [Citation129] as shown in .
Fig. 39 Mechanism of photostabilization of PVC through absorption of UV light and dissipation light energy as heat.
The 1,3,4-thiadiazole ring has two different atoms of different electronegativty such as nitrogen and sulfur. The polarity of this ring explains the attraction between the stabilizer and PVC, .
Fig. 40 Mechanism of photostabilization of PVC through the interaction between PVC and Schiff base compounds.
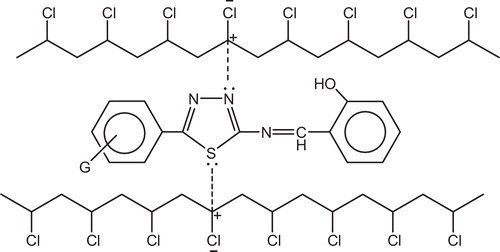
The synthesis of polymer-bound chelating ligands and the selective chelation of specific metal ions is a field of active research [Citation121]. Metal chelate complexes generally known as photostabilizers for PVC through both peroxide decomposer and excited state quencher.
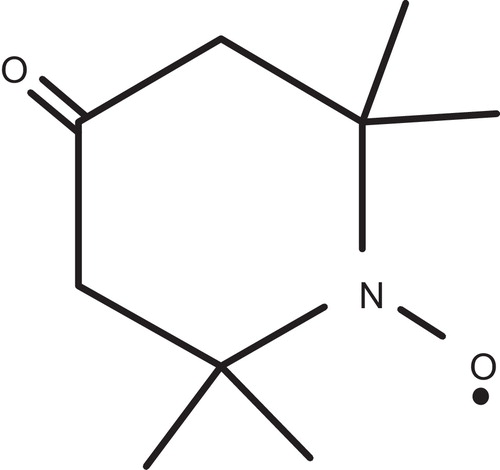
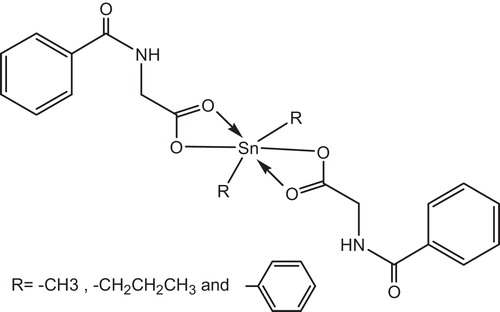
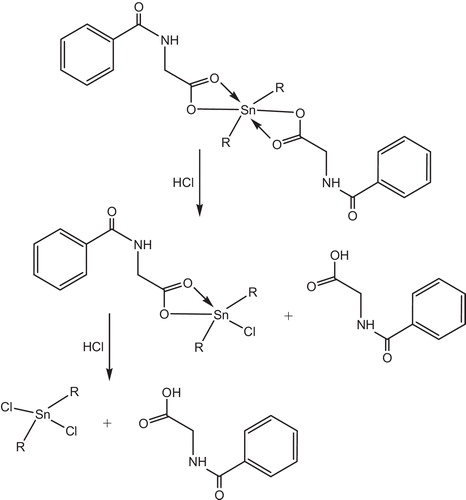
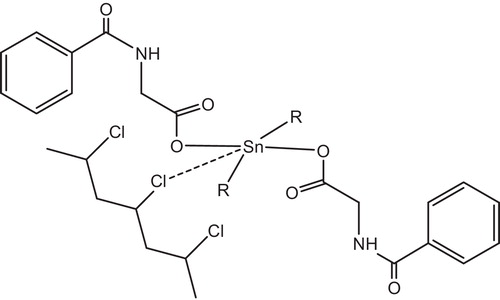
Yousif et al. had reported the photostabilizing effect of diorganotin(IV) complexes of the ligand benzamidoacetic acid complexes in PVC polymer, see . These additives stabilize the PVC films through HCl scavenging, UV absorption, peroxide decomposer and radical scavenger mechanisms [Citation11].
Tin carboxylates stabilize PVC by two mechanisms, depending on the metal. Strongly basic carboxylates, which have little or no Lewis acidity, are mostly HCl scavengers, .
IR spectroscopy has shown that metals carboxylates associate with PVC molecules at the surface of primary particles and are, consequently, very effective in the substitution of allylic chlorine. In this mechanism, the stabilizer is classified as a primary stabilizer. It has been postulated that metals stabilizers associate with chlorine atoms at the surface of PVC primary particles which explains their high efficiency in PVC stabilization [Citation130], .
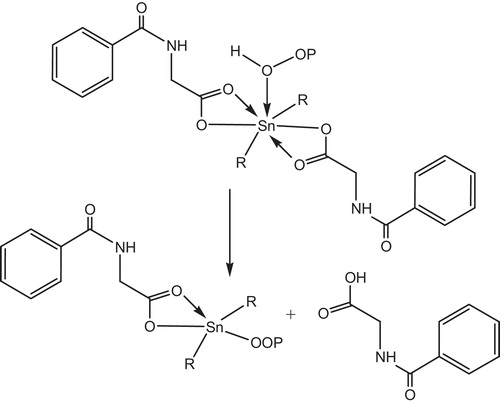
Metal chelate complexes generally known as photostabilizers for PVC through both peroxide decomposer and excited state quencher. Therefore, it is expected that these complexes act as peroxide decomposers through the following proposed mechanism, .
The effect of the tetradentate ligand [H2L] of the N2O2 type, N{2[3(1-carboxyiminoethyl)-1-phenyl]butilydene-2-amino propionic acid}, and its metal complexes with Cr(III), Fe(III), Cu(II) and Zn(II), , on the photodegradation of poly(vinyl chloride) films blended with a concentration range of 2–2.5% by weight of this complex was investigated [Citation131].
Fig. 45 Suggested geometrics and names of N{2[3(1-carboxyiminoethyl)-1-phenyl]butilydene-2-amino propionic acid}complexes.
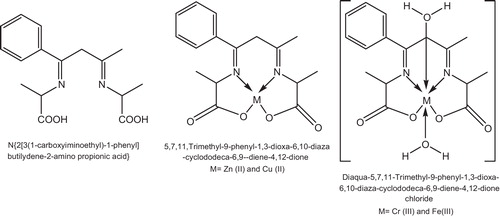
It has been found that Cr(III) and Fe(III) chelates enhanced the ratio of C–C cleavage via depression in the average molecular weight Mw of the studied PVC films. However the Zn(II) chelate increased the photostabilization of the polymer via lowering of the quantum yield ϕcs of the scissions and indices of carbonyls, hydroxyls and polyenes.
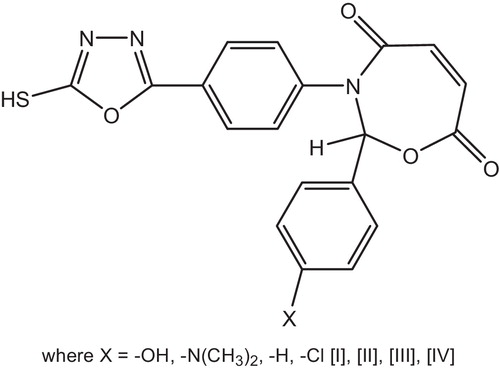
The photostabilization of PVC was studied using a new four heterocyclic compounds containing 1,3-oxazepine and 1,3,4-oxadiazole, see .
These compounds stabilize PVC by different mechanisms such as UV absorber, screener or by radical scavenger. These stabilizers provide good long-term stability and are usually referred to these mechanisms. The most probable mechanisms involved in a photostabilization is the hydroxyl group of the additive might acts as radical scavenger for photostabilization process. Therefore this Schiff bases, besides acting as UV absorber may also act as radical scavenger additives [Citation122].
Methyl methacrylate–butadiene–styrene (MBS) terpolymer has been developed for improving impact resistance of PVC [Citation132]. It also acts as a processing aid. The MBS resin is a graft polymer of styrene and methyl methacrylate onto the butadiene-styrene rubber, and it has a characteristic shell/core structure, which consists of a styrene-butadiene core and styrene-methacrylate shell that is compatible with PVC.
The influence of methyl methacrylate–butadiene–styrene copolymer (MBS) on poly(vinyl chloride) (PVC) photodehydrochlorination and photooxidative degradation was investigated by UV–vis, FTIR and fluorescence spectra. It was found that MBS decelerates PVC photodehydrochlorination and photocrosslinking; the effect is caused by the fact that the Cl radicals react with H atoms at tertiary atoms in polystyrene of MBS and initiate the depolymerization of the PMMA chains of MBS, then leads to reinitiation of PVC dehydrochlorination [Citation133].
Polymer modification can influence the properties of the macromolecule [Citation134]. Recently, scientists does able to modified PVC by introduction aromatic and heterocyclic moieties through halogen displacement reaction. PVC, thus modified, showed improved overall photochemical stability and optical properties. The facial chlorine displacement from PVC indicated the possibility on easy anchoring of ligands to PVC matrix. In view of paucity of any information on PVC in this line, we undertook the synthesis of PVC-heterocyclic compounds.
The influence of introducing benzothiazole and benzimidazole as a pending groups into the repeating unit of PVC has been studied on the bases of photostability measurements.
Yousif et al. prepared five modified polymers by the covalent modification of commercial PVC with benzothiazole and benzimidazole binding units.
These polymers also function as radical scavengers through energy transfer and by forming unreactive charge transfer complexes between the modified polymers and excited state of the chromophore (POO–) and stabilize through resonating structures, .
Fig. 47 Mechanism of photostabilization of PAA as radical scavengers through energy transfer and forming unreactive charge transfer and stabilize through resonating structure.
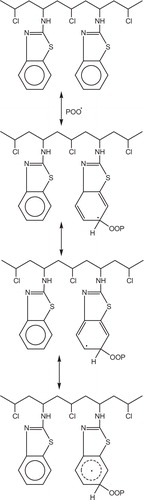
The UV light absorption by these polymers containing these heterocylic units dissipates the UV energy to harmless heat energy, .
27 Conclusion
In recent years, the use of polymeric materials has rapidly increased but it is well established that rapid photodegradation of these materials is possible when they are exposed to natural weathering.
This review was talk about the photostabilization and the effect of UV. Light on the photodegradation of poly(vinyl chloride). The “hydroperoxide” (POOH) is the most important initiator in the photooxidative process.
So most of the common polymers used in such applications contain photostabilizers to reduce photodamage and to ensure acceptable life times under outdoor exposure conditions. The photostabilization of polymers may be achieved in many ways. The following stabilizing systems have been developed, which depend on the action of stabilizer: light screeners, UV absorbers, excited-state quenchers, peroxide decomposers, and radical scavengers; of these, it is generally believed that excited-state quenchers, peroxide decomposers, and radical scavengers are the most effective.
However photoproduction will be enhanced if the additives can decompose –OOH, and possibly act as quenchers of some exited state in the early stages of the photodegradation.
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
EY and AH researched data and wrote the article. Both authors read and approved the final manuscript.
Acknowledgment
The authors acknowledge the Department of Chemistry, College of Science, Al-Nahrain University and CRDF for their encouragement.
Notes
Peer review under responsibility of Taibah University
References
- D.WilesPhotostabilization of macromolecules by excited state quenchingPure Appl. Chem.501978291297
- J.RabekPolymer Photodegradation – Mechanisms and Experimental Methods1995Chapman HallCambridge
- D.FeldmanPolymer weathering: photo-oxidationJ. Polym. Environ.102002163173
- A.AmeerB.AbdallhA.AhmedE.YousifSynthesis and characterization of polyvinyl chloride chemically modified by aminesOpen J. Polym. Chem.320131115
- K.MinskerS.KolesovG.ZaikovDegradation and Stabilization of Vinyl Chloride Based Polymers1988Pergamon PressOxford7882
- N.GrassieG.ScottPolymer Degradation and Stabilization1985Cambridge University PressLondon
- J.RabekB.RanbyStudied on the photooxidation mechanism of polymers, photolysis and photooxidation of polystyreneJ. Polym. Sci.121974273291
- J.RabekB.RanbyPolymer photophysics and photochemistry: an introduction to the study of photoprocess of macromoleculesJ. Polym. Sci.121974273281
- E.YousifR.HaddadPhotodegradation and photostabilization of polymers, especially polystyrene: reviewSpringer Open J.22013389430
- E.YousifA.HameedN.SalihJ.SalimonB.AbdullahNew photostabilizers for polystyrene based on 2,3-dihydro-(5-mercapto-1,3,4-oxadiazol-2-yl)-phenyl-2-(substituted)-1,3,4-oxazepine-4,7-dione compoundsSpringer Plus J.2201318
- E.YousifJ.SalimonN.SalihNew photostabilizer for PVC based on some diorganotin(IV) complexesJ. Saudi Chem. Soc.16201219
- E.YousifM.AbdallhH.HashimN.SalihJ.SalimonB.M.AbdullahY.WinOptical properties of pure and modified poly(vinyl chloride)Springer Plus J.4201318
- X.ZhangT.ZhaoH.PiS.GuoMechanochemical preparation of a novel polymeric photostabilizer for poly(vinyl chloride)J. Appl. Polym. Sci.116201030793086
- W.ShiJ.ZhangX.ShiG.JiangDifferent photodegradation processes of PVC with different average degrees of polymerizationJ. Appl. Polym. Sci.1072008528540
- A.AndradyS.HamidX.HuA.TorikaiEffects of increased solar ultraviolet radiation on materialsJ. Photochem. Photobiol. B46199896103
- H.AbbasPhotostabilization of Poly(Vinyl) Chloride by Bis(2-Amino Acetate Benzothiazole) Complexes (M.Sc. thesis)2008College of Science, Al Nahrain University
- R.BurgessManufacture and Processing of PVC2005Elsevier Applied ScienceLondon
- M.AkayIntroduction to Polymer Science and Technology2012Ventus Publishing ApSAnkara
- H.ZimmermannPoly(vinyl chloride) polymerization performance-enhancing initiators with emphasis on high activity grades and water-based dispersionsJ. Vinyl Addit. Technol.21996287294
- T.XieA.HamielecP.WoodD.WoodsSuspension, bulk, and emulsion polymerization of vinyl chloride-mechanism, kinetics, and reactor modellingJ. Vinyl Technol.131991225
- M.NaqviStructure and stability of polyvinyl chlorideJ. Macromol. Sci.-Rev. Macromol. Chem. Phys.251985119155
- E.YousifJ.SalimonN.SalihA.JawadY.WinNew stabilizers for PVC based on some diorganotin(IV) complexes with benzamidoleucineArab. J. Chem.5201218
- E.LinakChemical Industries Newsletter2009
- E.YousifPhotostabilization of PVC: Principles and Applications2012LAP, LAMBERTGermany
- G.ZaikovK.GumargalievaT.PokholokY.MoiseevV.ZaikovKinetic aspects of aging of poly(vinyl chloride)-based polymer materialsPolym.-Plast. Technol. Eng.3932000567650
- E.YousifM.AliwiA.AmeerJ.UkalImproved photostability of PVC films in the presence of 2-thioacetic acid-5-phenyl–1,3,4-oxadiazole complexesTurk. J. Chem.332009339410
- M.SabaaE.OrabyA.Abdel NabyR.MohamedN-phenyl-3-substituted 5-pyrazolone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photodegradationJ. Appl. Polym. Sci.101200615431555
- L.Pimentel RealA.FerrariaA.Botelho do RegoComparison of different photo-oxidation conditions of poly(vinyl chloride) for outdoor applicationsPolym. Testing272008743751
- E.YousifN.SalihJ.SalimonImprovement of the photostabilization of PVC films in the presence of 2N-salicylidene-5-(substituted)-1,3,4-thiadiazoleJ. Appl. Polym. Sci.120201122072214
- E.ArkişD.BalköseThermal stabilisation of poly(vinyl chloride) by organotin compoundsPolym. Degrad. Stab.8820044651
- C.MarvalJ.SampleM.RoyThe structure of vinyl polymers. VI. Polyvinyl halidesJ. Am. Chem. Soc.61193932413244
- D.BraunThermal degradation of polyvinyl chloridePure Appl. Chem.261971173192
- M.AsahinaM.OnozukaThermal decomposition of model compounds of polyvinyl chloride. I. Gaseous thermal decomposition of model compounds having secondary and tertiary chlorineJ. Polym. Sci., Part A2196435053513
- Z.MayerThermal decomposition of polyvinyl chloride and of its low-molecular-weight model compoundsJ. Macromol. Sci.-Rev. Macromol. Chem. Phys.111974263292
- N.BensemraT.HoangA.GuyotThermal dehydrochlorination and stabilization of poly(vinyl chloride) in solution: Part V. Influence of structural defects in the polymerPolym. Degrad. Stab.281990173184
- Z.VymazalZ.VymazalovaPhotodegradation of PVC stabilized by organotin compoundsEur. Polym. J.27199112651270
- T.HjertbergE.SörvikFormation of anomalous structures in PVC and their influence on the thermal stability: 2. Branch structures and tertiary chlorinePolymer241983673684
- V.PercecA.CappottoB.BarboiuMetal-catalyzed living radical graft copolymerization of butyl methacrylate and styrene initiated from the structural defects of narrow molecular weight distribution poly(vinyl chloride)Macromol. Chem. Phys.203200216741683
- L.JianZ.DafeiZ.DerenThe photo-degradation of PVC: Part I-Photo-degradation in air and nitrogenPolym. Degrad. Stab.301990335343
- M.VeronelliM.MauroS.BresadolaInfluence of thermal dehydrochlorination on the photooxidation kinetics of PVC samplesPolym. Degrad. Stab.661999349357
- L.JianZ.DafeiZ.DerenThe photo-degradation of PVC: Part II-Structural changes in PVC chainsPolym. Degrad. Stab.31199117
- Q.YuS.ZhuW.ZhouPeroxide induced crosslinking and degradation of polyvinyl chlorideJ. Polym. Sci., Part A: Polym. Chem.361998851860
- J.BauerA.SabelThe influence of oxygen on the polymerization of vinyl chloride in the example, the suspension polymerizationAngew. Makromol. Chem.4719751527
- S.CrawleyI.McNeillPreparation and degradation of head-to-head PVCJ. Polym. Sci.: Polym. Chem. Ed.16197825932606
- T.RadiotisG.BrownComputer simulations of microstructure changes resulting from the thermal degradation of PVCJ. Macromol. Sci. Pure Appl. Chem.341997743757
- F.CastilloG.MartinezR.SastreJ.MillanV.BellengerB.GuptaJ.VerduInfluence of structure on the photo-degradation of PVC. Part IV-A conclusive approach to the mechanism of photo-oxidation and photo-dehydrochlorinationPolym. Degrad. Stab.271990111
- T.HjertbergA.WendelReduction of poly(vinyl chloride) with tri-n-butyltin hydridePolymer23198216411645
- K.AbbåsBranching in poly(vinyl chloride)J. Macromol. Sci.-Phys.B141977159166
- W.StarnesF.SchillingK.AbbåsI.PlitzR.HartlessF.BoveyStructural selectivities in the reduction of poly(vinyl chloride) with lithium aluminum hydride and tri-n-butyltin hydrideMacromolecules1219791319
- W.StarnesB.WojciechowskiA.VelaquezG.BenediktMolecular microstructure of the ethyl branch segments in poly(vinyl chloride)Macromolecules25199236383642
- T.HjertbergE.SörvikFormation of anomalous structures in poly(vinyl chloride) and their influence on the thermal stability. Effect of polymerization temperature and pressure;P.P.KlemchukPolymer Stabilization and Degradationvol. 2801985American Chemical SocietyWashington, DC259284
- T.HjertbergE.SörvikFormation of anomalous structures in PVC and their influence on the thermal stability: 3. Internal chloroallylic groupsPolymer241983685692
- W.StarnesV.ZaikovH.ChungB.WojciechowskiV.TranK.SaylorG.BenediktIntramolecular hydrogen transfers in vinyl chloride polymerization: routes to doubly branched structures and internal double bondsMacromolecules31199815081517
- W.StarnesStructural and mechanistic aspects of the thermal degradation of poly(vinyl chloride)Progr. Polym. Sci.27200221332170
- E.YousifA.AhmedM.MahmoudNew Organic Photo-Stabilizers for Rigid PVC Against Photodegradation2012LAP, LAMBERTGermany
- A.Shànchez-SolisA.PadillaEffect of sands on poly(vinyl chloride) resistance to ultraviolet lightPolym. Bull.361996753758
- N.GhatgeS.VernekarEvaluation of ultraviolet light absorbers in poly vinyl chloride (PVC) Part IIAngew. Makromol. Chem.201971175180
- A.GonzalezJ.PastorJ.De SajaF.de laMonitoring the UV degradation of PVC window frames by microhardness analysisJ. Appl. Polym. Sci.38198918791882
- L.AudouinC.Anton-PrinetJ.VerduG.MurM.GayThickness distribution of degradation products during photochemical aging of rigid PVCAngew. Makromol. Chem.26119982534
- C.Anton-PrinetG.MurM.GayL.AudouinJ.VerduPhotoageing of rigid PVC-III. Influence of exposure conditions on the thickness distribution of photoproductsPolym. Degrad. Stab.601998283289
- A.TorikaiH.HasegawaAccelerated photodegradation of poly(vinyl chloride)Polym. Degrad. Stab.631999441445
- B.SinghN.SharmaMechanistic implications of plastic degradationPolym. Degrad. Stab.932008561584
- J.RabekPolymer Degradation Mechanisms and Experimental Methods1994SpringerStockholm
- C.DeckerM.BalandierPhoto-oxidation of poly(vinyl chloride)Polym. Photochem.11981221232
- Y.LiuW.LiuM.HouMetal dicarboxylates as thermal stabilizers for PVCPolym. Degrad. Stab.92200715651571
- J.SummersE.RabinovitchThe chemical mechanisms of outdoor weathering in polyvinyl chlorideJ. Vinyl Technol.5319839195
- J.GardetteJ.LemairePhotothermal and thermal oxidations of rigid plasticised and pigmented poly(vinyl chloride)Polym. Degrad. Stab.341991135167
- J.GardetteJ.LemairePrediction of the long-term outdoor weathering of poly(vinyl chloride)J. Vinyl Technol.151993113118
- J.GardetteJ.LemaireReversible discoloration effects in the photo aging of poly(vinyl chloride)J. Vinyl Addit. Technol.31997107110
- E.YousifA.HameedSynthesis and photostability study of some modified poly(vinyl chloride) containing pendant benzothiazole and benzimidozole ringInt. J. Chem.2120106580
- J.RabekB.RanbyPhotodegradation, Photooxidation and Photostabilization of Polymers1975WileyNew York
- C.DeckerDegradation of poly(vinyl chloride) by UV radiation—II: mechanismEur. Polym. J.201984149155
- J.AhamadD.JohnDetermination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper(II) oxide and copper(II) chlorideE-J. Chem.632009685692
- K.MinskerG.FedoseyevaDegradation and Stabilization of Polyvinylchloride1979KhimiaMoscow (in Russian)
- J.LabartaM.HerreroP.TiembloC.MijangosH.ReineckeWetchemical surface modification of plasticized PVC. Characterization by FTIR-ATR and Raman microscopyPolymer44200322632269
- M.KratzD.HendrickerPreparation of polymer-bound 2,2′-dipyridylamine and some of its transition metal complexesPolymer27198616411643
- E.YousifJ.SalimonN.SalihB.AbdullahPhotochemical study of PS based on presence Schiff of 2,5-dimercapto-1,3,4-thiadiazole as photostabilizer6th International Engineering Conference, Energy and Environment2013
- S.ChmelaP.LajoieP.HrdlovicJ.LacosteCombined oligomeric light and heat stabilizersPolym. Degrad. Stab.712001171177
- E.YousifE.BakirJ.SalimonN.SalihEvaluation of Schiff bases of 2,5-dimercapto-1,3,4-thiadiazole as photostabilizer for poly(methyl methacrylate)J. Saudi Chem. Soc.162012279285
- N.SalihJ.SalimonE.YousifA.HamedMicrowave synthesis of some new 1,3-oxazepine compounds as photostabilizing additives for poly(vinyl chloride) filmsAsian J. Chem.2512201367486754
- N.CheremisionHand Book of Engineering Polymeric Materials, New York1997 (Chapter 8)
- S.L.DavidY.G.HsuanAssessing the photodegradation of geosynthetics by outdoor exposure and laboratory weatherometerGeotext. Geomembr.212003111122
- A.FrankH.LeonardL.RobertA.JosephJ.DennisUV stabilizerEncycl. Polym. Sci. Technol.82002269309
- P.KlemchukM.GandeE.CordolaHindered amine mechanisms: Part III-Investigations using isotopic labellingPolym. Degrad. Stab.2719906574
- G.GueskensM.KandaG.NedelkosThe mechanism of action of hindered amine stabilizers. Production of nitroxy radicals and inhibition of the photo oxidation in different polymersBull. Soc. Chim. Belg.99199010851100
- E.StepN.TurroM.GandeP.KlemchukMechanism of polymer stabilization by hindered-amine light stabilizers (HALS). Model investigations of the interaction of peroxy radicals with HALS amines and amino ethersMacromolecules27199425292539
- J.StretanskiA New Light Stabilizer for Polyolefine, SPE-RETEC Polyolefins III1981
- CabotCarbon Blacks for Protection of Plastics Exposed to Ultraviolet Light, CABOT Technical Report S-1141990Cabot CorporationBillerica, MA7
- T.TurtonJ.WhiteEffect of stabilizer and pigment on photo-degradation depth profiles in polypropylenePolym. Degrad. Stab.742001559568
- D.OlsonS.SchroeterUV screen progenitors thermally labile urethane derivatives of hydroxyphenylbenzotriazoles and hydroxybenzophenonesJ. Appl. Polym. Sci.22197821652176
- A.LamollaEnergy Transfer and Organic Photochemistry, Technique of Organic Chemistry1969WileyNew York114 (Chapter 2)
- H.HellarH.BlattmanSome aspects of stabilization of polymers against lightPure Appl. Chem.361973141162
- S.BeavanD.PhillipsCollisional effects on the phosphorescence of 1,1,1-trifluoroacetone vapourJ. Photochem.81978247261
- E.DanA.SomersallJ.GuilletPhotochemistry of ketone polymers. IX. Triplet energy transfer in poly(vinyl ketones)Macromolecules61973228230
- G.GeuskensG.NedelkosThe oxidation of hindered amine light stabilizers to nitroxy radicals in solution and in polymersPolym. Degrad. Stab.191987365
- P.GijsmanJ.HennekensD.TummersThe mechanism of action of hindered amine light stabilizersPolym. Degrad. Stab.391993225233
- F.GugumusCurrent trends in mode of action of hindered amine light stabilizersPolym. Degrad. Stab.401993167215
- T.KurumadaH.OhsawaT.FujitaT.TodaThe effect of N-substituents of hindered amine on photo-oxidation of polypropyleneJ. Polym. Sci., Polym. Chem. Ed.221984277281
- B.FelderPolymer Stabilization and Degradation2801985American Chemical SocietyWashington, DC69
- D.BauerJ.GerlockPhoto-stabilization and photo-degradation in organic coatings containing a hindered amine light stabilizer: Part IV-Photo-initiation rates and photo-oxidation rates in unstabilized coatingsPolym. Degrad. Stab.271990271284
- J.GerlockD.MielewskiD.BauerPhoto-initiation rate behavior of weathered coatings: ESR-nitroxide decay assayPolym. Degrad. Stab.201988123134
- D.BauerM.DeanJ.GerlockComparison of photostabilization in acrylic/urethane and acrylic/melamine coatings containing hindered amines and ultraviolet absorbersInd. Eng. Chem. Res.2719886570
- M.NeimanE.RozantzevY.MamedovaFree radical reactions involving no unpaired electronsNature1961962472
- I.VulicS.SammuelsA.WagnerNew Breakthroughs in Hindered Amine Light Stabilizer PerformanceADDCON WORLD; 2, Addcon World Conference1999
- J.DurmisD.CarlssonK.ChanD.WilesPhotostabilization of polypropylene by a hindered amineJ. Polym. Sci. Polym. Lett. Ed.191981549554
- E.RozantsevP.LoshadkinThe history and modern problems of free radical chemistry. 100 years of free radical chemistryDes. Monom. Polym.42001281300
- M.DankoŠ.ChmelaP.HrdlovičPhotochemical stability and photostabilizing efficiency of anthracene/hindered amine stabilizers in polymer matricesPolym. Degrad. Stab.792003333343
- H.HellarH.BlattmanSome aspects of the light protection of polymersPure Appl. Chem.301972145166
- J. Stretanski, Polyolefins stabilized against light-induced degradation, U.S. Pat. 4,237,042 (to American Cyanamid Co.) (1980).
- J. Stretanski, F. Loffelman, Stabilized titanium dioxide-pigmented polyolefin compositions, U.S. Pat. 4,670,491 (to American Cyanamid Co.) (1987).
- N.AllenM.MudherP.GreenPhoto-stabilising action of ortho-hydroxy aromatic compounds in polypropylene film: UV absorption versus radical scavengingPolym. Degrad. Stab.719848394
- E.YousifPhotostabilization of PVC by Inorganic Complexes2010LAP, LAMBERT, Academic PublishingGermany
- E.YousifPhotostabilization of Thermoplastic Polymers2012Lambert, Academic PublishingGermany
- L.DavidY.GraceAssessing the photo-degradation of geosynthetics by outdoor exposure and laboratory weatherometerGeotext. Geomembr.212003111122
- Y.HsuanR.KoernerAntioxidant depletion lifetime in high density polyethylene geomembranesJ. Geotechn. Geo-environ. Eng., ASCE12461998532541
- P.GijsmanJ.HennekensK.JanssenComparison of UV degradation chemistry in accelerated (Xenon) aging tests and outdoor test (II)Polym. Degrad. Stab.4619946374
- P.GijsmanA.DozemanComparison of the UV-degradation chemistry of unstabilized and HALS-stabilized polyethylene and polypropylenePolym. Degrad. Stab.5319964550
- R.BacalogluM.FischDegradation and stabilization of poly(vinyl chloride). V. Reaction mechanism of poly(vinyl chloride) degradationPolym. Degrad. Stab.4719953357
- B.IvanThermal stability, degradation, and stabilization mechanisms of poly(vinyl chloride)R.CloughN.BillinghamK.GillenPolymer Durability, Degradation, Stabilization, and Lifetime Prediction, Advances in Chemistry Series 2491996American Chemical SocietyWashington, DC1933
- E.YousifR.HaddadA.AhmedPhotodegradation and Photostabilization of Polystyrene2013LAP, LAMPERTGermany
- E.YousifJ.SalimonN.SalihNew stabilizer for polystyrene based on 2-thioacetic acid benzothiazol complexesJ. Appl. Polym. Sci.125201219221927
- R.RasheedH.MansoorE.YousifA.HameedY.FarinaA.GraisaPhotostabilizing of PVC films by 2-(aryl)-5-[4-(aryloxy)-phenyl]-1,3,4-oxadiazole compoundsEur. J. Sci. Res.302009464477
- J.GardetteS.GaumetJ.PhilippartInfluence of experimental conditions on the photo-oxidation of PVCJ. Appl. Poylm. Sci.4811199318851895
- E.YousifS.HameedE.BakirSynthesis and photochemical study of poly(vinyl chloride)-1,3,4-oxadiazole and 1,3,4-thiadiazoleJ. Al-Nahrain Univ.1012007712
- S.RabieA.KhalilAntimicrobial agents as photostabilizers for rigid poly(vinyl chloride)Polym. Adv. Technol.32201213941402
- D.BraunS.RabieN.KhaireldinM.Abd El-GhaffarPreparation and evaluation of some benzophenone terpolymers as photostabilizers for rigid PVCJ. Vinyl Addit. Technol.17201147155
- S.RabieA.KhalilA.NadaDiamide derivatives as photostabilizers for plasticized poly(vinyl chloride)J. Vinyl Addit. Technol.142008191196
- E.YousifTriorganotin(IV) complexes photo-stabilizers for rigid PVC against photodegradationJ. Taibah Univ. Sci.720137987
- P.SimonL.ValkoKinetics of polymer degradation involving the splitting off of small molecules: Part 6-Dehydrochlorination of PVC in an atmosphere of HClPolym. Degrad. Stab.351992249253
- E.YousifN.SalihJ.SalimonImprovement of the photostabilization of PVC films in the presence of thioacetic acid benzothiazole complexesMalay. J. Anal. Sci.15120118192
- N.MahmoudA.SalamM.WessalA.MohammedSpectroscopic study of the effect of a new metal chelate on the stability of PVCJ. Assoc. Arab Univ. Basic Appl. Sci.1420136774
- E.CrawfordA.LesserMechanics of rubber particle cavitation in toughened polyvinylchloride (PVC)Polymer41200058655870
- X.ChenJ.WangJ.ShenEffect of UV-irradiation on poly(vinyl chloride) modified by methyl methacrylate–butadiene–styrene copolymerPolym. Degrad. Stab.872005527533
- Z.GenhuaC.PanPreparation of star polymers based on polystyrene or poly(styrene-b-N-isopropyl acrylamide) and divinylbenzene via reversible addition-fragmentation chain transfer polymerizationPolymer46200528022810