 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Three species of oyster mushrooms (Pleurotus spp.) that are cultivated mostly throughout the year in the plains of India were studied for their nutritional value and their antioxidant properties. Highest protein content was found in Pleurotus florida (22–25% dw) followed by Pleurotus citrinopileatus (20–22% dw) and Pleurotus pulmonarius (15–18% dw). Cholesterol content was in the range of 0.6–0.8% dw, making them low-cholesterol, proteinaceous food. The antioxidant properties of the three species were of both enzymatical and non-enzymatical nature. Reducing power, chelating activity on Fe2+ and total phenol contents were higher in P. florida than in P. pulmonarius and P. citrinopileatus. With regard to antioxidative enzymes, P. florida had the highest peroxidase and superoxide dismutase activity whereas maximum catalase activity was found in P. pulmonarius. P. florida had higher antioxidative activity than P. pulmonarius and P. citrinopileatus thereby highlighting its nutraceutical values along with nutritional qualities.
Abbreviations:
1 Introduction
Oxidation is essential for all living organisms for the production of energy to fuel their biological processes. However, oxygen-centred free radicals and other reactive oxygen species that are continuously being produced in vivo may result in cell death and tissue damage. Oxidative damage caused by these free radicals may be related to ageing and diseases, such as atherosclerosis, diabetes, cancer and cirrhosis [Citation1]. Almost all organisms are well equipped with several defence systems against free-radical damage by oxidative enzymes such as superoxide dismutase (SOD) and catalase (CAT), or by chemical compounds such as α-tocopherol, ascorbic acid, carotenoids, polyphenolic compounds and glutathione [Citation2,Citation3]. These systems are insufficient to prevent damage entirely [Citation4]. However, antioxidant supplements or antioxidant-containing foods are important in the human diet to prevent or to reduce oxidative damage [Citation5,Citation6]. The restriction in the use of synthetic antioxidants, such as BHA (2-tert-butyl-4-methoxyphenol) and BHT (2,6-ditert-butyl-4-methylphenol), has led to an increased interest in natural antioxidant substances [Citation7,Citation8]. Natural antioxidants are being extensively studied for their properties to protect organisms and cells from damage brought about by oxidative stress, the latter being considered a cause of ageing and degenerative diseases. The antioxidants present in foods, especially vegetables, are phenolic compounds (phenolic acids and flavonoids), carotenoids, tocopherol and ascorbic acid [Citation5,Citation9], which are important protective agents for human health [Citation10,Citation11].
Mushrooms are rich sources of those compounds [12–15Citation[12] Citation[13] Citation[14] Citation[15]] and their activities are determined by spectrophotometric techniques [16–18Citation[16] Citation[17] Citation[18]]. Progressively, electrochemical techniques have been developed and tested as an alternative tool for the evaluation of different food extracts, expressed in terms of ‘antioxidant power’, due to their quickness, simplicity and low cost [19–23Citation[19] Citation[20] Citation[21] Citation[22] Citation[23]].
Substances that may be considered a food or part of a food and that provide medical or health benefits like the prevention and treatment of diseases are referred to as nutraceuticals [Citation24,Citation25]. Mushrooms have become attractive as a functional food and as a source for the development of drugs and nutraceuticals [Citation5,Citation18,Citation26] due to their antioxidant [Citation12,Citation27], antitumor [Citation28] and antimicrobial properties [Citation13,Citation29]. Besides their pharmacological features [Citation30], mushrooms are becoming more important in our diet thanks to their nutritional value, which is related to high protein and low fat/energy contents [Citation31,Citation32]. Mushrooms accumulate a variety of secondary metabolites, including phenolic compound, polyketides, terpenes, variegatic acid, diboviquinone and steroids [Citation33,Citation34]. Most of their antioxidative potential is the result of the redox properties of phenolic compounds that allow them to act as reducing agents, hydrogen donors and singlet oxygen quenchers [Citation35].
The study included three species of Pleurotus: P. florida (Mont.) Singer, P. pulmonarius (Fr.) Quel. and P. citrinopileatus Singer. These mushrooms, which are cultivated mostly throughout the year in the plains of India, were studied for their nutritional value (protein and cholesterol content) and their enzymatic and non-enzymatic antioxidative properties in order to establish their potential for human consumption and health.
2 Materials and methods
2.1 Mushrooms
Strains of Pleurotus florida (Mont.) Singer (Khatun, 02-11), Pleurotus pulmonarius (Fr.) Quel. (Khatun, 03-11) and Pleurotus citrinopileatus Singer (Khatun, 01-11) were obtained from the National Centre for Mushroom Research and Training, Solan, India and were maintained on potato dextrose agar (PDA) slants. Spawn was produced by growing mycelium on wheat grain in autoclavable polypropylene bags (15 cm × 12 cm) following standard methods [Citation36]. Rice straw and water hyacinth were used as substrates for growing mushrooms. The aerial parts (stems and leaves only) were sun dried; the roots were discarded as these were reported to absorb heavy metals [Citation37]. Both substrates were chopped into small pieces (3–5 cm) and steeped overnight in tap water. After draining the excess water the substrates were pasteurized at 80 °C for 90 min. After cooling down, 2 kg of moistened substrate was transferred to transparent polythene bags, 50 cm × 35 cm, perforated with 20–25 evenly distributed holes. The substrates were used either alone (rice straw or water hyacinth) or in combination (rice straw + water hyacinth). The bags were then inoculated with wheat spawns at 5% (w/w) on a wet weight basis following multilayer technique and incubated in the semi-dark at temperatures conditioned between 26 °C and 28 °C during winter (December to February) and pre-summer (February to April) seasons. After a spawn run period of 13–19 days (according to substrate combination), the polythene bags were removed. The temperature and relative humidity (RH) inside the cropping room were then maintained at 18–22 °C/24–28 °C and 75–80%/80–85%, respectively, during fruit body formation.
Fresh fruiting bodies of the three Pleurotus species, cultivated at the Mycology and Plant Pathology Laboratory, Department of Botany, Burdwan University, were cleaned and washed with distilled water. They were cut into small pieces, dried to a constant weight in a hot-air oven at 60 °C (moisture content for P. florida 82.67 ± 8.1% fw, for P. pulmonarius 80.57 ± 9.5% fw and for P. citrinopileatus 79.27 ± 11.7% fw), ground to a particle size of 40 mesh by using mortar and pestle and stored at −4 °C in airtight containers until further use.
2.2 Protein
Protein content of the mushrooms was measured according to Bradford [Citation38]. Dry (20 mg) tissue was crushed with 2.5 mL methanol and a pinch of neutral sand and centrifuged at 5000 rpm for 15 min. The pellet was taken discarding the supernatant. The pellet added with 2.5 mL of 1 M NaOH was kept at 80 °C for 1 h and cooled at room temperature. The volume was adjusted to the initial level and centrifuged at 10,000 rpm for 15 min. One tenth of a mL supernatant mixed with 5 mL Bradford reagent [Citation38] was used for measuring protein content. After 5 min, absorbance was recorded at 595 nm in a UV–vis spectrophotometer (Systronics 117) against a control sample.
2.3 Cholesterol
Cholesterol content of the mushrooms was measured following the CHOD/PAP method [Citation39] using a diagnostic kit (Crest Biosystem, Goa, India).
2.4 Catalase
To measure catalase (CAT) content 100 mg of dry mushroom tissue was crushed in a mortar and pestle with 3 mL of phosphate buffer (0.1 M, pH 7.0) at 1–4 °C and centrifuged at 3000 rpm for 15 min. The supernatant was analysed for the enzyme following the method of Luck [Citation40]. The enzyme activity was expressed as units per g dry tissue.
2.5 Peroxidase
Peroxidase activity was estimated following the method of Mahadevan and Sridhar [Citation41] using freshly prepared pyrogallol reagent. The reaction mixture containing 5 mL of freshly prepared pyrogallol reagent (prepared by mixing 10 mL of 0.5 M pyrogallol solution and 12.5 mL of 0.66 M phosphate buffer made up with distilled water to 100 mL) and 1.5 mL of the enzyme extract was placed in a spectrophotometer tube and immediately adjusted to zero absorbance in a spectrophotometer. Half of a mL of 1% H2O2 solution was added and the contents were mixed by inverting the tube. The reaction was initiated by the addition of H2O2. Enzyme activity was recorded as the change in absorbance per min at 430 nm immediately after the addition of substrate and was expressed as the change in absorbance per min per g dry tissue.
2.6 Superoxide dismutase
Superoxide dismutase (SOD) activity was estimated following the method of Kakkar et al. [Citation42] using Na-pyrophosphate buffer (0.052 M; pH 8.3). The extract was obtained by centrifuging at 10,000 rpm for 15 min. The supernatant was recovered and kept in a tube in an ice bath until further use. Fifty μL enzyme extract, 1.35 mL of double-distilled water, 1.2 mL Na-pyrophosphate buffer, 0.1 mL phenozinemethosulphate and 0.3 mL nitrobluetetrazolium were mixed. Then 0.2 mL NADH was added to initiate the reaction followed by incubation at 39 °C for 90 s. The reaction was terminated by adding 1 mL glacial acetic acid. Four mL n-butanol was added and mixed vigorously in a Vortex mixer and centrifuged at 4000 rpm for 10 min. The upper layer of butanol was taken off and reading was taken at 560 nm against a corresponding blank solution. The assay was based on chromogen production using phenozinemethosulphate and nitrobluetetrazolium in the presence of SOD. One unit of SOD activity is defined as the amount of enzyme that inhibits the rate of reaction by 50% under specified conditions. The enzyme activity was expressed as units per g dry tissue.
2.7 Reducing power and chelating activity on Fe2+
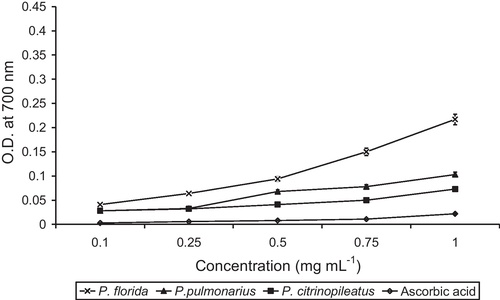
To measure the reducing power and chelating activity on Fe2+ 1 g of dry mushroom powder was stirred in 50 mL ethanol and water (3:1, v/v) at 75 °C and centrifuged at 3000 rpm for 1 h. The extract was then filtered using Whatman No. 1 filter paper, the filtrate was collected and dried in a rotary evaporator at 40 °C, transferred into a plastic bottle and stored at −20 °C until further use. The reducing power of the ethanol extract was measured using potassium ferricyanide [Citation43]. The presence of reductants (antioxidants) in the samples would result in reducing Fe3+/ferricyanide complex to the ferrous form (Fe2+). The Fe2+ was monitored by measuring the formation of Perl's Prussian blue at 700 nm [Citation44]. The reducing power of the ethanolic extract was compared with that of ascorbic acid.
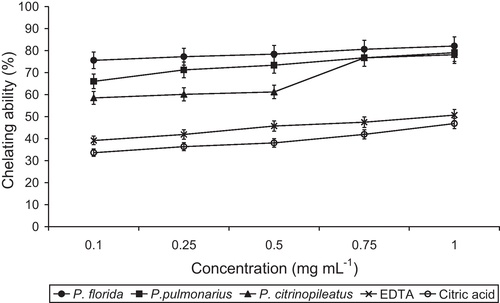
The chelating activity of the ethanolic extracts on Fe2+ was measured using the ferrozine reagent [Citation45]. An aliquot of 1 mL of different concentrations (0.1, 0.25, 0.50, 0.75 and 1.00 mg mL−1) of the ethanolic extracts was mixed with 2.5 mL of de-ionized water. The mixture was left to react with FeCl2 (2 mM, 0.1 mL) and ferrozine (5 mM, 0.2 mL) for 10 min at room temperature. The absorbance was measured at 562 nm in a UV–vis spectrophotometer (Systronics 117). A lower absorbance indicates a higher chelating power. Chelating activity on Fe2+ of the ethanolic extract was compared with that of ethylenediaminetetraacetic acid (EDTA) and citric acid. Chelating activity was calculated following the equation:
2.8 Total phenol
One hundred mg dry mushroom tissue was placed in 5–10 mL of 80% ethyl alcohol, boiled for 5–10 min in a hot water bath (100 °C) and then cooled in a pan of cold water (20 °C) for 30 min. The tissue was then crushed in a mortar and pestle for 5–10 min and passed through a double-layered cloth. The crushed tissue was again extracted by boiling in 80% alcohol, cooled and then passed through Whatman No. 1 filter paper. Total phenol was estimated using Folin–Ciaocalteu reagent [Citation41]. Catechol was used as the standard. The amount of phenolics was expressed as μg catechol g−1 dry tissue.
2.9 Statistical analysis
Data were expressed as means ± standard deviation. Statistically significant differences among the means were determined using one-way ANOVA (p < 0.01). Correlations between two antioxidant attributes were determined by Pearson Correlation analysis using Statistical Package for the Social Sciences (SPSS) version 17.0 statistics software.
3 Results and discussion
The results revealed that among the nutritional properties, protein content remained significantly higher (p < 0.01) in P. florida (23.8 ± 1.30% dw) than in P. citrinopileatus (20.8 ± 0.84% dw) and P. pulmonarius (16.8 ± 1.30% dw). The insignificant cholesterol contents (p > 0.05) of the three mushroom species (P. florida 0.68 ± 0.08% dw, P. pulmonarius 0.7 ± 0% dw, P. citrinopileatus 0.8 ± 0% dw) make them a low cholesterol, protein-rich food. Generally, high protein and low fat levels were also described by previous authors [Citation31,Citation32,Citation46,Citation47]. Oyster mushrooms (Pleurotus spp.) are excellently edible and nutritious, and rank among the most widely cultivated mushrooms in the world [Citation48].
As regards the enzymatic antioxidant properties, catalase (CAT) activity () was found to be significantly higher (p < 0.01) in P. pulmonarius than in P. citrinopileatus and P. florida. Peroxidase and superoxide dismutase (SOD) activities and total phenol content were significantly higher (p < 0.01) in P. florida than in the two other species. Antioxidants are needed by the human body to combat free radical activities responsible for accelerating ageing processes of tissues and pathologies such as cancer or cardiovascular diseases. Endogenous supply of antioxidants such as CAT, SOD and peroxidase protects the cells against excessive levels of free radicals [Citation49]. The extracts from fruiting bodies of P. florida and P. pulmonarius occurring in India had significant antioxidant properties [Citation50,Citation51]. Ramesh and Pattar [Citation52] observed that P. pulmonarius occurring in Western Ghats of Karnataka, India had significant antioxidant properties and antimicrobial activities. Ethanol extracts from the mycelium of Pleurotus sajor-caju, P. florida and P. aureovillosus have been investigated for their antioxidant capacity, antimicrobial activities and phytochemicals. The levels of phenolic compounds in ethanol extract of P. sajor-caju, P. florida and P. aureovillosus were found to be 6.001 ± 0.04 μg mg−1, 7.501 ± 0.10 μg mg−1 and 6.72 ± 0.05 μg mg−1, respectively [Citation53]. The phenolic compounds may contribute directly to antioxidative action [Citation54]. Lee et al. [Citation55] showed that in P. citrinopileatus, phenols were the major antioxidant components and their contents were found in the order: fruiting bodies (8.62–12.38 mg g−1) > mycelia grown on fermentation broth (5.84–7.85 mg g−1) > fermentation filtrate (4.80–5.57 mg g−1). The antioxidant properties of compounds are well correlated with the contents of phenolic compounds [Citation56]. Phenols are reported to have good antioxidant [Citation57], antimutagenic and anticancer properties [Citation58]. So, the high total phenol content in P. florida in the present study is most likely responsible for its better antioxidant properties.
Table 1 Enzymatic antioxidant properties and total phenol content of Pleurotus florida, Pleurotus pulmonarius and Pleurotus citrinopileatus. Values expressed as means ± standard deviation (n = 5).
Our results () show that the reducing power of the three mushroom extracts increased significantly (p < 0.01) with an increase in total phenol content: 0.217, 0.103 and 0.073 A700, at 1.0 mg mL−1 in P. florida, P. pulmonarius and P. citrinopileatus, respectively. In addition, this parameter was higher for the three mushrooms extracts than for ascorbic acid. The reducing power of the three extracts from fruiting bodies of P. citrinopileatus was reported to be 1.03–1.10 at 5 mg mL−1, which was higher than those of mycelia and filtrate [Citation55]. On the other hand, the ethanolic extract of Ganoderma lucidum and Cordyceps sinensis was reported to have a reducing power of 2.50 and 1.97, respectively, at 1.0 mg mL−1 [Citation59]. These values were higher than those obtained in the present study for the three mushrooms. Reductants such as ascorbic acid can react directly with peroxides and also with certain precursors and thereby prevent the formation of peroxide [Citation60]. The reducing power of various extracts might be due to its hydrogen donating ability [Citation60]. So, P. florida, P. pulmonarius and P. citrinopileatus might contain reductants that could react with free radicals to stabilize and terminate free radical chain reactions [Citation61,Citation62].
The mushroom extracts showed a chelating activity on Fe2+ that depended on the concentration (). The chelating activity on Fe2+ of the P. florida extract was higher than that of the P. pulmonarius and P. citrinopileatus extracts. The chelating activities of the individual mushroom extracts with a concentration of 1.0 mg mL−1 (82.07%, 79.04% and 78.14%, respectively) were significantly higher (p < 0.01) than that of EDTA (50.66%) and citric acid (46.84%). The chelating abilities of P. citrinopileatus in extracts of ethanol, cold water and hot water at 5 mg mL−1 were reported to be 46%, 67% and 82%, respectively [Citation55]. The present findings are in line with the results found by Lee et al. [Citation55] in ethanolic extract of P. citrinopileatus.
The antioxidant potential of the two commonly used edible mushrooms P. florida and P. eous was estimated by Imran et al. [Citation63]. P. florida had a higher chelating activity against ferrous ions than P. eous which corroborates the present findings. Thus the study suggests that the consumption of oyster mushrooms may enhance the immune power of our body against diseases due to free radicals. So they can be used as a dietary supplement along with other foods or as a drug itself.
Chelation property may provide protection against oxidative damage and iron-overload [Citation64]. The chelating ability of a plant extract provides a strategy to avoid free-radical generation and iron-overload through the chelation of metal ion [Citation65]. Since ferrous ions are the most effective pro-oxidants in food systems [Citation66], the high ferrous ion chelating properties of the extracts of P. florida would make consumption of this mushroom beneficial.
Chelating ability was positively correlated with CAT for P. florida (r = 0.925; p < 0.05) with total phenol for P. pulmonarius (r = 0.907, p < 0.05) and with peroxidase (r = 0.879; p < 0.05), CAT (r = 0.879; p < 0.05) and total phenol (r = 0.932; p < 0.05) for P. citrinopileatus. Peroxidase was also positively correlated with CAT (r = 1.000; p < 0.01).
The phenolics of the three Pleurotus mushrooms showed a high tendency of chelation particularly for iron, leading to the stabilization and termination of radical chain reactions [67–69Citation[67] Citation[68] Citation[69]].
4 Conclusion
Of the three Pleurotus spp. studied, P. florida has a higher protein content and a much better antioxidative action than P. pulmonarius and P. citrinopileatus; highlighting its nutraceutical value. The Pleurotus mushrooms are comparable to the best antioxidants, which is attributed to their catalase, phenolics and peroxidase contents.
Acknowledgement
The UGC, Government of India, New Delhi, is acknowledged for its financial support of the first author by granting him a Meritorious Fellowship.
References
- B. Halliwell J.M.C. Gutteridge Oxygen toxicity, oxygen radicals, transition metals and disease Biochemical Journal 219 1984 1 4
- E. Niki H. Shimaski M. Mino Antioxidantism Free Radical and Biological Defense 1994 Gakkai Syuppan Center Tokyopp. 3–16
- J.L. Mau H.C. Lin S.F. Song Antioxidant properties of several specialty mushrooms Food Research International 35 2002 519 526
- M.G. Simic Mechanisms of inhibition of free-radical processed in mutagenesis and carcinogenesis Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis 202 1988 377 386
- M. Elmastas O. Isildak I. Turkekul N. Temur Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms Journal of Food Composition and Analysis 20 2007 337 345
- B. Halliwell J.M.C. Gutteridge Free Radicals in Biology and Medicine 2003 Oxford University Press Oxford, UKpp. 617–783
- B.M. Ames Dietary carcinogens and anticarcinogens: oxygen radical and degenerative disease Science 221 1983 1256 1263
- A.L. Branen Toxicology and biochemistry of butylated hydroxy anisole and butylated hydroxy toluene Journal of the American Oil Chemists Society 52 1975 59 63
- R. Cazzi R. Ricardy T. Aglitti V. Gatta P. Petricone R. De Salvia Ascorbic acid and b-carotene as modulators of oxidative damage Carcinogen 18 1997 223 228
- G. Block B. Patterson A. Subar Fruits, vegetables and cancer prevention: a review of the epidemiological evidence Nutrition and Cancer 18 1992 1 29
- M.W. Gillman A.L. Cupples D. Gagnon B.M. Posner R.C. Ellison W.P. Castelli A.P. Wolf Protective effect of fruits and vegetables on development of stroke in men JAMA-Journal of the American Medical Association 273 1995 1113 1117
- L. Barros M.J. Ferreira B. Queiros I.C.F.R. Ferreira P. Baptista Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities Food Chemistry 103 2007 413 419
- L. Barros R.C. Calhelha J.A. Vaz I.C.F.R. Ferreira P. Baptista L.M. Entevinho Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts European Food Research and Technology 225 2007 151 156
- P. Valentao P.B. Andrade J. Rangel B. Ribeiro B.M. Silva P. Baptista R.M. Seabra Effect of the conservation procedure on the contents of phenolic compounds and organic acids in Chanterelle (Cantharellus cibarius) mushroom Journal of Agricultural Food Chemistry 53 2005 4925 4931
- P. Valentao G. Lopes M. Valente P. Barbosa P.B. Andrade B.M. Silva P. Baptista R.M. Seabra Quantification of nine organic acids in wild mushrooms Journal of Agricultural and Food Chemistry 53 2005 3626 3630
- L. Barros P. Baptista D.M. Correia J.S. Morais I.C.F.R. Ferreira Effects of conservation treatment and cooking on the chemical composition and antioxidant activity of Portuguese wild edible mushrooms Journal of Agricultural and Food Chemistry 55 2007 4781 4788
- L. Barros P. Baptista I.C.F.R. Ferreira Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays Food and Chemical Toxicology 45 2007 1731 1737
- I.C.F.R. Ferreira P. Baptista M. Vilas-Boas L. Barros Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity Food Chemistry 100 2007 1511 1516
- A.J. Blasco M.C. Gonzalez A. Escarpa Electrochemical approach for discriminating and measuring predominant flavonoids and phenolic acids using differential pulse voltammetry: towards an electrochemical index of natural antioxidants Analytica Chimica Acta 511 2004 71 81
- A.J. Blasco M.C. Rogerio M.C. Gonzalez A. Escarpa “Electrochemical index” as a screening method to determine “total polyphenolics” in foods: a proposal Analytica Chimica Acta 539 2005 237 244
- S. Chevion M.A. Roberts M. Chevion The use of cyclic voltammetry for the evaluation of antioxidant capacity Free Radical Biology and Medicine 28 2000 860 870
- M.S. Cosio S. Buratti S. Mannino S. Benedetti Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family Food Chemistry 97 2006 725 731
- E.I. Korotkova Y.A. Karbainov A.V. Shevchuk Study of antioxidant properties by voltammetry Journal of Electroanalytical Chemistry 518 2002 56 60
- Y.Z. Fang S. Yang G. Wu Free radicals, antioxidants, and nutrition Nutrition 18 2002 872 879
- P. Furst K.S. Kuhn Fish oil emulsions: what benefits can they bring? Clinical Nutrition 19 2000 7 14
- N. Caglarirmak The nutrients of exotic mushrooms (Lentinula edodes and Pleurotus species) and an estimated approach to the volatile compounds Food Chemistry 105 2007 1188 1194
- K.M. Lo P.C.K. Cheung Antioxidant activity of extracts from the fruiting bodies of Agrocybe aegerita var. Alba Food Chemistry 89 2005 533 539
- S.P. Wasser A.L. Weis Medicinal properties of substances occurring in higher Basidiomycetes mushrooms: current perspectives (review) International Journal of Medicinal Mushrooms 1 1999 31 62
- L. Barros D.M. Correira I.C.F.R. Ferreira P. Baptista C.S. Buelga Optimization of the determination of tocopherols in Agaricus sp. edible mushrooms by a normal phase liquid chromatographic method Food Chemistry 110 2008 1046 1050
- U. Lindequist T.H.J. Niedermeyer W.D. Julich The pharmacological potential of mushrooms Evidence-Based Complementary and Alternative Medicine 2 2005 285 299
- D. Agahar–Murugkar G. Subbulakshmi Nutritional value of edible wild mushrooms collected from the Khasi hills of Meghalaya Food Chemistry 89 2005 599 603
- L. Barros P. Baptista D.M. Correia S. Casal B. Oliveira I.C.F.R. Ferreira Fatty acid and sugar compositions, and nutritional value of five wild edible mushrooms from Northeast Portugal Food Chemistry 105 2007 140 145
- P.L. Teissedre N. Landrault Wine phenolics: contribution to dietary intake and bioavailability Food Research International 33 2000 461 467
- L.M. Cheung P.C.K. Cheung V.E.C. Ooi Antioxidant activity and total phenolics of edible mushroom extracts Food Chemistry 81 2003 249 255
- C.A. Rice-Evans N. Miller G. Paganga Antioxidant properties of phenolic compounds Trends in Plant Science 2 1997 152 159
- B.C. Suman V.P. Sharma Steps in mushroom growing B.C. Suman V.P. Sharma Mushroom Cultivation and Uses 2007 Agrobios India 70 74
- A.J.C. Kelly J.K. Uno Curtis C.L. Burman Spectroscopic studies of the interaction of EU(III) with the roots of water hyacinth Water Air Soil Pollution 119 2004 171 176
- M.M. Bradford A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding Analytical Biochemistry 72 1976 48 254
- P. Roeschlau E. Bernt W. Gruber Enzymatic determination of total cholesterol in serum Zeitschrift für Klinische Chemie und Klinische Biochemie 12 1974 p226
- H. Luck H.U. Bergmeyer Methods of Enzymatic Analysis 2nd edition 1965 Academic Press New York and London 895 897
- A. Mahadevan R. Sridhar Methods in Physiological Plant Pathology 2nd edition 1982 Sivakami Publications Madras, India
- P. Kakkar B. Das P.N. Viswanathan A modified spectrophotometric assay of SOD Indian Journal of Biochemistry and Biophysics 21 1984 130 132
- M. Oyaizu Studies on products of browning reaction: antioxidant activities of products of browning reaction prepared from glucosamine Japanese Journal of Nutrition 44 1986 307 315
- Y.C. Chung C.T. Chang W.W. Chao C.F. Lin S.T. Chou Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK 1 Journal of Agricultural and Food Chemistry 50 2002 2454 2458
- E.A. Decker B. Welch Role of ferritin as a lipid oxidation catalyst in muscle food Journal of Agricultural and Food Chemistry 38 1990 674 677
- C.M. Ude A.E.N. Ezenwugo R.C. Agu Composition and food value of sclerotium (osu) and edible mushroom (Pleurotus tuber-regium) Journal of Food Science and Technology 38 2001 612 614
- V.A. Diez A. Alvarez Compositional and nutritional studies on two wild edible mushrooms from northwest Spain Food Chemistry 75 2001 417 422
- S.T. Chang Global impact edible and medicinal mushrooms on human welfare in the 21st century: non-green evolution International Journal of Medicinal Mushrooms 1 1999 1 7
- V.O. Oyetayo Mushrooms indigenous to Nigeria as potential source of myconutraceuticals—a review Current Trends in Biotechnology and Pharmacy 2 2008 471 477
- T.A. Ajith K.K. Janardhanan Indian medicinal mushrooms as a source of antioxidant and antitumor agents Journal of Clinical Biochemistry and Nutrition 40 2007 157 162
- B. Lakshmi J.C. Tilak S. Adhikari T.P.A. Devasagayam K.K. Janardhanan Evaluation of antioxidant activity of selected Indian mushrooms Pharmaceutical Biology 42 2004 179 185
- C. Ramesh M.G. Pattar Antimicrobial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of Western Ghats of Karnataka, India Pharmacognosy Research 2 2010 107 112
- K.J. Loganathan S. Ramalingam V. Venkatasubbu K. Venkatesan Studies on the phytochemical, antioxidant and antimicrobial properties of three indigenous Pleurotus species Journal of Molecular Microbiology and Biotechnology 1 2008 20 29
- M. Tanaka C.W. Kuei Y. Nagashima T. Taguchi Application of antioxidative Maillrad reaction products from histidine and glucose to sardine products Nippon Suisan Gakkaishil 54 1998 1409 1414
- Y.L. Lee G.W. Huang Z.C. Liang J.L. Mau Antioxidant properties of three extract from Pleurotus citrinopileatus LWT-Food Science and Technology 40 2007 823 833
- Y.S. Velioglu G. Mazza L. Gao B.D. Oomah Antioxidant activity and total phenolics in selected fruits, vegetables and grain products Journal of Agricultural and Food Chemistry 46 1998 4113 4117
- O.U. Orhan Determination of antioxidant and acetylcholinesterase inhibitory potentials and total phenol contents of selected polypore mushrooms Journal of Food Composition and Analysis 24 3 2011 386 390
- N. Ahmad H. Mukhtar Green tea polyphenols and cancer: Biological mechanisms and practical implications Nutrition Reviews 57 1999 78 83
- R.P. Singh K.K. Mishra R.C. Verma S. Tandon A. Dubey Anti-oxidative properties of Ganoderma lucidum and Cordyceps sinensis Mushroom Research 16 2007 19 22
- K. Shimada K. Fujikawa K. Yahara T. Nakamura Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion Journal of Agricultural and Food Chemistry 40 1992 945 948
- H.D. Belitz W. Grosch P. Schieberle Food Chemistry 3rd edition 2004 Spinger Verlag GmbH Berlinpp. 157–242
- J.D. Bruijn C. Loyola P. Aqueveque J. Canumir M. Cortez A. France Antioxidant properties of extracts obtained from Grifola gargal mushrooms Micologia Aplicada International 21 2009 11 18
- M.M. Imran M.M. Raja M.A. Basith J.A. Asarudeen Determination of total phenol, flavonoid and antioxidant activity of edible mushrooms Pleurotus florida and Pleurotus eous International Food Research Journal 18 2011 579 582
- L.S. Lai S.T. Chan W.W. Chao Studies on the antioxidative activities of mesona procumbers Hems/leaf gum Journal of Agricultural and Food Chemistry 19 2001 963 968
- J. Robak R. Marankiewicz Scavenging of reactive oxygen species as the mechanism of drug action Polish Journal of Pharmacology 47 1995 89 98
- R. Yamaguchi M.A. Tatsumi K. Kato U. Yoshimitsu Effect of metal salts and fructose on the autoxidation of methyl linoleate in emulsions Agricultural and Biological Chemistry 52 1988 849 850
- H.J. Jung H.J. Park R.G. Kim K.M. Shin J. Ha W. Choi H.J. Kim K.T. Lee In vivo anti-inflammatory and antinoceptive effects of Liriodendrin isolated from the stem of Acanthopanax senticasus Planta Medica 69 2003 610 616
- A. Michalak Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress Polish Journal of Environmental Studies 15 2006 523 530
- S. Srivastava P. Goyal Novel biomaterials Decontamination of Toxic Metals from Waste Water 2010 Springer-Verlag Berlin/Heidelberg
