Abstract
Capsule The Skylark Alauda arvensis had the highest overall mortality in ten Northern Portuguese wind farms surveyed between 2006 and 2011. Analysis from the integration of conventional and molecular techniques suggest a sex and age biased mortality affecting mainly adult males (90.9%), which may be related to their characteristic breeding male song-flights making them highly vulnerable to collision with wind turbines. The results highlight the added value of more complete population impact assessments that go beyond simple carcass identification at wind farms.
The exploitation of renewable energy sources is rapidly increasing as a result of several governmental strategies to produce ‘clean energy’ aimed at reducing pollution effects, consumption and overexploitation of fossil natural resources (Panwar et al. Citation2011). In this context, investment in wind energy worldwide has grown significantly over the last decade (Kaldellis & Zafirakis Citation2011) and the impacts of wind farms on biodiversity have already been discussed in a wide variety of studies (Kuvlesky et al. Citation2007, Santos et al. Citation2010, Northrup & Wittemyer Citation2013), with emphasis on the amount of birds and bats killed by collision with wind turbines (Barclay et al. Citation2007, Kunz et al. Citation2007, Tellería Citation2009). Concerning birds, wind farms may entail different impacts (e.g. collision fatalities, habitat loss and fragmentation, displacement/avoidance and exclusion from breeding and foraging areas), affecting a wide range of resident and migratory species (Drewitt & Langston Citation2006, Farfán et al. Citation2009, Pearce-Higgins et al. Citation2012). Although previous studies performed in Northern and Central regions of Portugal have shown that mortality by collision with wind turbines is particularly high for a resident species, the Skylark Alauda arvensis (Linnaeus 1758), little is known about the repercussions on the life cycle dynamics and trends of the affected bird populations (Bernardino et al. Citation2010, Citation2012, Pearce-Higgins et al. Citation2012).
The Skylark is a common resident or altitudinal short-distance migratory bird, breeding in most European countries (Cramp Citation1988). In Northern and Central Portugal, this species is mainly observed in highlands (over 800 m) from spring to autumn, particularly during the breeding season (March–July). The environmental conditions associated with altitude are important factors that influence local Skylark population densities and seasonal distribution (Donald Citation2004), forcing population displacement to lowlands during the wintering period (Catry et al. Citation2010). Despite the ‘least concern’ (LC) conservation status of Skylark in Portugal, this species exhibits worldwide decreasing trends (IUCN Citation2012).
The determination of the incidence of differential mortality by sex and/or age induced by wind turbines may provide additional important data for conservation and management purposes (Ewen et al. Citation2001, Buenestado et al. Citation2009) by improving our understanding of the potential consequences for the fitness of populations affected by wind turbine collisions (Schmickl & Karsai Citation2010, Rymešová et al. Citation2012). Nevertheless, sex and age analysis of birds killed at wind farms has only been performed in a few studies and the carcasses have only been occasionally submitted to necropsy examinations to determine/confirm the cause of death (Stienen et al. Citation2008, Bevanger et al. Citation2010). In this context, the main goal of the present research was to combine necropsy examinations, morphological and histological analysis with molecular sexing techniques in order to confirm differential mortality according to age or sex in Skylark carcasses found and collected at wind farms in North Portugal.
This study was based on bird mortality detected at ten Northern Portuguese wind farms from monitoring surveys carried out between January 2006 and December 2011 (). These wind farms are located in the mountain areas of ‘Serra do Marão’ (29T 593364E 4566887N), ‘Serra do Alvão’ (29T 598869E 4580026N) and ‘Serra de Montemuro’ (29T 581372E 4535224N), representing a total of 88 wind turbines installed at altitudes ranging from 950 to 1340 m, where the dominant habitats are heathlands. The mortality surveys (see for sampling period details) were carried out using spiral transects within a maximum radius of 60 m from the wind turbine (Erickson et al. Citation2000). All carcasses detected were identified (when possible), photographed, georeferenced, catalogued and stored. In the laboratory, bird species identification was confirmed, and then specimens were submitted to a necropsy exam and tissues were stored at –20°C for subsequent molecular analysis.
Table 1. Characterization of monitoring periods and parameters used to estimate the real mortality of Skylark Alauda arvensis in the wind farms under study.
Morphological analysis was performed in order to confirm the species and age of the individuals, by using reference guides (Cramp Citation1988, Svensson Citation1992). Thus, bird plumage was examined in order to distinguish breeding individuals and juveniles based on the feather shape and condition. Necropsy exams were carried out to determine the gender and if mortality was consistent with a collision with wind turbines. Most of the birds showed traumatic injuries, such as skull and rib fractures, subcutaneous and/or muscular haemorrhages. In general, carcasses showed advanced decomposition of internal organs or were totally or partially mummified, which hindered sex identification, especially in young specimens and/or carcasses severely contaminated with larval insects in the coelomic cavity. Samples from reproductive organs were collected for histological analysis, to confirm the gender (whenever it was not possible to do so macroscopically) and to establish sexual gonadal maturation (Bacha & Bacha Citation2000, Samour Citation2008). Molecular genetic approaches were used to overcome the limitations of the aforementioned techniques for sex identification of carcasses in advanced stages of decomposition. DNA was isolated from muscle tissues using a conventional salting out protocol (Morinha et al. Citation2013). Molecular sexing of all carcasses was performed based on the chromodomain helicase DNA-binding protein 1 (CHD1) gene, using the primers P2 (5′-TCTGCATCGCTAAATCCTTT-3′) and P8 (5′-CTCCCAAGGATGAGRAAYTG-3′) as described by Griffiths et al. (Citation1998). Polymerase chain reaction (PCR) amplifications were performed in a volume of 10 μl, containing 5 μl of 2x MyTaq HS Mix (Bioline), 2.5 pmol of each primer and 20 ng of genomic DNA. The thermal protocol consisted of an initial denaturation at 95°C for 5 minutes followed by 30 cycles of 95°C for 30 seconds, 50°C for 1 minute, 72°C for 30 seconds and a final extension at 60°C for 10 minutes. PCR products were separated by electrophoresis on 2.5% agarose gels stained with ethidium bromide.
The Skylark was the species with the highest collision mortality incidence (37.3%) among all bird carcasses (n = 59) found in the mortality surveys carried out in the wind farms under study (). The other bird species killed by collision with wind turbines were Delichon urbicum (32.2%), Apus apus (5.1%), Circus pygargus (5.1%), Falco tinnunculus (3.4%), Sylvia undata (3.4%), Phylloscopus spp. (3.4%), Turdus merula (3.4%), Regulus ignicapilla (1.7%), Saxicola torquatus (1.7%), Ficedula hypoleuca (1.7%) and Emberiza cia (1.7%).
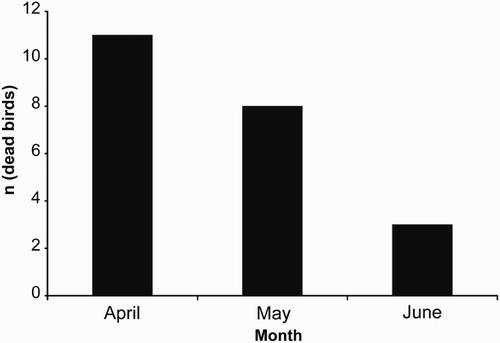
The analysis of the 22 Skylark carcasses collected, revealed a clear mortality incidence on adult males (90.9%), suggesting the occurrence of a non-random differential mortality, both in sex and age. Skylark carcasses were mainly found between April and May (), which coincides with the main breeding season of the species. Skylark males perform a characteristic song flight associated with mate choice and territoriality (Hedenström Citation1995, Donald Citation2004) and this may lead to sex-biased mortality at wind farms. This behaviour involves a vertical ascending flight, ranging from 50 m to more than 200 m in some exceptional cases (Hedenström Citation1995, Donald Citation2004), which would likely represent a high risk of collision in the presence of wind turbines. This sex-biased mortality may have important consequences to population dynamics if it reduces local reproductive success. The occurrence of non-random sex mortality has been reported for several bird species (Martín et al. Citation2007, Rymešová et al. Citation2012), and may be a critical factor in small isolated populations (Stifetten & Dale Citation2006). For Skylark populations, the high mortality of adult males at the beginning of the breeding season could decrease the number of established breeding pairs, which could affect the reproductive success of the local populations. Although most Skylark populations have a number of unmated males (the floaters), which may then occupy the breeding territories made available due to accidental mortality of the occupant male (Donald Citation2004), a recurrent and almost exclusive high mortality of males in mountain areas with several wind farms seems likely to affect the recruitment of new males and so population stability. The potential total number of Skylark fatalities was calculated for the spring season by using two complementary estimators (Korner-Nievergelt et al. Citation2011, Bastos et al. Citation2013), considering several relevant parameters such as monitoring periodicity, monitoring period, number of wind turbines, mean persistence of carcasses, mean searcher efficiency and detected mortality. The real mortality estimated was one order of magnitude higher when compared with the original results obtained for the detected mortality (). Therefore, persistent long-term impacts at these levels may have potential effects on demographics, abundance, genetic structure and gene pool quality of the affected populations (National Research Council (NRC) Citation2007).
Figure 1. Monthly cumulative distribution of fatalities for the Skylark Alauda arvensis based on carcasses collected at wind farms between 2006 and 2011.
Since this type of induced differential mortality on Skylark may have distinct ecological consequences, depending on the local population fitness and the prevailing environmental conditions (Bjørnstad et al. Citation1999, Devereux et al. Citation2008, Pearce-Higgins et al. Citation2009, Citation2012), an integrated analysis of distribution patterns, main habitats used, phenological aspects, ecological requirements and the risk associated with behavioural characteristics may be crucial to understanding the real magnitude of the impacts attributable to wind farms (Fox et al. Citation2006, Santos et al. Citation2010). Despite this, most studies on wind farms have disregarded these approaches, especially in the case of ‘common’ species, and many criticisms have arisen from lack of scientific rigour and failure to evaluate effective impacts on bird populations (Masden et al. Citation2010). This study highlights the potential of detailed analyses of bird carcasses detected at wind farms for more conclusive studies by the combination of post-mortem examinations and molecular techniques that allow the collection of more comprehensive information about the potential impacts of wind farms, particularly for bird species where sex cannot be readily determined from visual examination. Overall, the data provided in this kind of study could be useful as the basis or as a complement to population trends forecasting, and to improve conservation and management strategies in specific case studies. Further multidisciplinary research is essential to assess the cumulative impact of differential mortality by sex and age at wind farms on the structure and dynamics of affected bird populations, such as in this case of the Skylark.
ACKNOWLEDGEMENTS
We thank all the members of Laboratory of Applied Ecology involved in the mortality surveys performed between 2006 and 2011.
FUNDING
This study was supported by funding from several ecological monitoring projects of the Laboratory of Applied Ecology (University of Trás-os-Montes e Alto Douro) and by Portuguese Foundation for Science and Technology (FCT) through the Ph.D. [grant number SFRH/BD/77872/2011].
REFERENCES
- Bacha, W. Jr & Bacha, L.M. 2000. Colour Atlas of Veterinary Histology, 2nd edn. Lippincott Williams & Wilkins, Philadelphia.
- Barclay, R.M.R., Baerwald, E.F. & Gruver, J.C. 2007. Variation in bat and bird fatalities at wind energy facilities: assessing the effects of rotor size and tower height. Can. J. Zool. 85: 381–387. doi: 10.1139/Z07-011
- Bastos, R., Santos, M. & Cabral, J.A. 2013. A new stochastic dynamic tool to improve the accuracy of mortality estimates for bats killed at wind farms. Ecol. Indicat. 34: 428–440. doi: 10.1016/j.ecolind.2013.06.003
- Bernardino, J., Mesquita, S., Marques, T., Cordeiro, A., Silva, M., Mascarenhas, M. & Costa, H. 2010. Bird and Bat Mortality Data in Portuguese Wind Farms – A Cumulative Analysis of 5 Years of Monitoring Surveys, Wind Wildlife Research Meeting VIII, Lakewood, Colorado.
- Bernardino, J., Zina, H., Passos, I., Costa, H., Fonseca, C., Pereira, M.J. & Mascarenhas, M. 2012. Bird and bat mortality at Portuguese wind farms. IAIA12 Conference Proceedings, 32nd Annual Meeting of the International Association for Impact Assessment, Porto, Portugal.
- Bevanger, K., Berntsen, F., Clausen, S., Dahl, E.L., Flagstad, Ø., Follestad, A., Halley, D., Hanssen, F., Johnsen, L., Kvaløy, P., Lund-Hoel, P., May, R., Nygård, T., Pedersen, H.C., Reitan, O., Røskaft, E., Steinheim, Y., Stokke, B. & Vang, R. 2010. Pre- and post-construction studies of conflicts between birds and wind turbines in coastal Norway (BirdWind). Report on findings 2007–2010. NINA Report 620. Norwegian Institute for Nature Research, Trondheim.
- Bjørnstad, O.N., Ims, R.A. & Lambin, X. 1999. Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends Ecol. Evol. 14: 427–431. doi: 10.1016/S0169-5347(99)01677-8
- Buenestado, F., Ferreras, P., Blanco-Aguiar, J.A., Tortosa, F.S. & Villafuerte, R. 2009. Survival and causes of mortality among wild Red-legged Partridges Alectoris rufa in southern Spain: implications for conservation. Ibis 151: 720–730. doi: 10.1111/j.1474-919X.2009.00952.x
- Catry, P., Costa, H., Elias, G. & Matias, R. 2010. Aves de Portugal. Ornitologia do Território Continental. Assírio & Alvim, Lisboa.
- Cramp, S. 1988. The Birds of the Western Palearctic, Vol. 5. Oxford University Press, Oxford.
- Devereux, C.L., Denny, M.J.H. & Whittingham, M.J. 2008. Minimal effects of wind turbines on the distribution of wintering farmland birds. J. Appl. Ecol. 45: 1689–1694. doi: 10.1111/j.1365-2664.2008.01560.x
- Donald, P.F. 2004. The Skylark. T & AD Poyser, London.
- Drewitt, A.L. & Langston, R.H.W. 2006. Assessing the impacts of wind farms on birds. Ibis 148: 29–42. doi: 10.1111/j.1474-919X.2006.00516.x
- Erickson, W.P., Johnson, G.D., Strickland, M.D. & Kronner, K. 2000. Avian and bat mortality associated with the Vansycle Wind Project, Umatilla County, Oregon: 1999 study year. Technical Report prepared by WEST, Inc. for Umatilla County Department of Resource Services and Development, Pendleton, Oregon.
- Ewen, J.G., Clarke, R.H., Moysey, E., Boulton, R.L., Crozier, R.H. & Clarke, M.F. 2001. Primary sex ratio bias in an endangered cooperatively breeding bird, the black-eared miner, and its implications for conservation. Biol. Conserv. 101: 137–145. doi: 10.1016/S0006-3207(01)00022-2
- Farfán, M.A., Vargas, J.M., Duarte, J. & Real, R. 2009. What is the impact of wind farms on birds? A case study in southern Spain. Biodivers. Conserv. 18: 3743–3758. doi: 10.1007/s10531-009-9677-4
- Fox, A.D., Desholm, M., Kahlert, J., Christensen, T.K. & Krag Petersen, I. 2006. Information needs to support environmental impact assessment of the effects of European marine offshore wind farms on birds. Ibis 148: 129–144. doi: 10.1111/j.1474-919X.2006.00510.x
- Griffiths, R., Double, M.C., Orr, K. & Dawson, R.J.G. 1998. A DNA test to sex most birds. Mol. Ecol. 7: 1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x
- Hedenström, A. 1995. Song flight performance in the Skylark Alauda arvensis. J. Avian Biol. 26: 337–342. doi: 10.2307/3677050
- IUCN, 2012. IUCN red list of threatened species. Version 2012.2. Available from: http://www.iucnredlist.org. [accessed 31 October 2013].
- Kaldellis, J.K. & Zafirakis, D. 2011. The wind energy (r)evolution: a short overview of a long history. Renew. Energ. 36: 1887–1901. doi: 10.1016/j.renene.2011.01.002
- Korner-Nievergelt, F., Korner-Nievergelt, P., Behr, O., Niermann, I., Brinkmann, R. & Hellriegel, B. 2011. A new method to determine bird and bat fatality at wind energy turbines from carcass searches. Wildl. Biol. 17: 350–363. doi: 10.2981/10-121
- Kunz, T.H., Arnett, E.B., Erickson, W.P., Hoar, A.R., Johnson, G.D., Larkin, R.P., Strickland, M.D., Thresher, R.W. & Tuttle, M.D. 2007. Ecological impacts of wind energy development on bats: questions, research needs, and hypotheses. Front. Ecol. Environ. 5: 315–324. doi: 10.1890/1540-9295(2007)5[315:EIOWED]2.0.CO;2
- Kuvlesky, W.P., Brennan, L.A., Morrison, M.L., Boydston, K.K., Ballard, B.M. & Bryant, F.C. 2007. Wind energy development and wildlife conservation: challenges an opportunities. J. Wildl. Manage. 71: 2487–2498. doi: 10.2193/2007-248
- Martín, C.A., Alonso, J.C., Alonso, J.A., Palacín, C., Magaña, M. & Martín, B. 2007. Sex-biased juvenile survival in a bird with extreme size dimorphism, the Great Bustard Otis tarda. J. Avian Biol. 38: 335–346. doi: 10.1111/j.2007.0908-8857.03811.x
- Masden, E.A., Fox, A.D., Furness, R.W., Bullman, R. & Haydon, D.T. 2010. Cumulative impact assessments and bird/wind farm interactions: developing a conceptual framework. Environ. Impact Assess. Rev. 30: 1–7. doi: 10.1016/j.eiar.2009.05.002
- Morinha, F., Travassos, P., Seixas, F., Santos, N., Sargo, R., Sousa, L., Magalhães, P., Cabral, J.A. & Bastos, E. 2013. High-resolution melting analysis for bird sexing: a successful approach to molecular sex identification using different biological samples. Mol. Ecol. Resour. 13: 473–483. doi: 10.1111/1755-0998.12081
- National Research Council (NRC), 2007. Environmental Impacts of Wind-Energy Projects, The National Academies Press, Washington, DC.
- Northrup, J.M. & Wittemyer, G. 2013. Characterising the impacts of emerging energy development on wildlife, with an eye towards mitigation. Ecol. Lett. 16: 112–125. doi: 10.1111/ele.12009
- Panwar, N.L., Kaushik, S.C. & Kothari, S. 2011. Role of renewable energy sources in environmental protection: a review. Renew. Sust. Energ. Rev. 15: 1513–1524. doi: 10.1016/j.rser.2010.11.037
- Pearce-Higgins, J.W., Stephen, L., Langston, R.H.W., Bainbridge, I.P. & Bullman, R. 2009. The distribution of breeding birds around upland wind farms. J. Appl. Ecol. 46: 1323–1331.
- Pearce-Higgins, J.W., Stephen, L., Douse, A. & Langston, R.H.W. 2012. Greater impacts of wind farms on bird populations during construction than subsequent operation: results of a multi-site and multi-species analysis. J. Appl. Ecol. 49: 386–394. doi: 10.1111/j.1365-2664.2012.02110.x
- Rymešová, D., Šmilauer, P. & Šálek, M. 2012. Sex – and age-biased mortality in wild Grey Partridge Perdix perdix populations. Ibis 154: 815–824. doi: 10.1111/j.1474-919X.2012.01259.x
- Samour, J. 2008. Avian Medicine, 2nd edn. Mosby/Elsevier, Edinburgh.
- Santos, M., Bastos, R., Travassos, P., Bessa, R., Repas, M. & Cabral, J. 2010. Predicting the trends of vertebrate species richness as a response to wind farms installation in mountain ecosystems of northwest Portugal. Ecol. Indicat. 10: 192–205. doi: 10.1016/j.ecolind.2009.04.014
- Schmickl, T. & Karsai, I. 2010. The interplay of sex ratio, male success and density-independent mortality affects population dynamics. Ecol. Modell. 221: 1089–1097. doi: 10.1016/j.ecolmodel.2009.12.028
- Stienen, E.W.M., Courtens, W., Everaert, J. & van de Walle, M. 2008. Sex-biased mortality of common terns in wind farm collisions. Condor 110: 154–157. doi: 10.1525/cond.2008.110.1.154
- Stifetten, Ø. & Dale, S. 2006. Viability of an endangered population of ortolan buntings: the effect of a skewed operational sex ratio. Biol. Conserv. 132: 88–97. doi: 10.1016/j.biocon.2006.03.016
- Svensson, L. 1992. Identification Guide to European Passerines, 4th edn. Märstatryck, Stockholm.
- Tellería, J.L. 2009. Wind power plants and the conservation of birds and bats in Spain: a geographical assessment. Biodivers. Conserv. 18: 1781–1791. doi: 10.1007/s10531-008-9558-2
