ABSTRACT
Capsule: Despite its population increase, the White-tailed Eagle Haliaeetus albicilla has not become food-limited, and does not pose a serious threat to birds of conservation concern in the region east of the Baltic Sea.
Aims: We aimed to test variations in the main prey groups and dietary niche breadth of White-tailed Eagles through the years and along an environmental gradient as well as to evaluate the extent of predation on bird species of conservation concern.
Methods: Prey remains were collected during nestling ringing from successful nests located in Lithuania.
Results: The frequency of the prey groups varied along an environmental gradient, with a general pattern of increasing avian prey consumed by pairs breeding in areas with less abundant aquatic habitats. The frequency of alternative prey (i.e. terrestrial birds, mammals) and dietary niche breadth did not increase between 2005 and 2018 and during which time the White-tailed Eagle population nearly doubled. Instead, the occurrence of Northern Pike Esox lucius remains, which was the most frequent single prey species, increased throughout the study period. Bird species of conservation concern constituted only a small fraction of all the prey identified.
Conclusions: The White-tailed Eagle has not become more dependent on alternative prey despite its population increase.
Food is an essential resource for organism survival and reproduction, thus it plays the keystone role in population regulation (Newton Citation1980, Citation2003), especially for apex predators, which are relatively free from predation. Knowledge on diets is necessary for understanding demographic rates in predators and is vital in setting conservation and/or habitat management objectives (Delibes-Mateos et al. Citation2007, Nadjafzadeh et al. Citation2015, Preston et al. Citation2017). In addition, most apex predator populations have recently recovered (Chakarov & Krüger Citation2010, Fasce et al. Citation2011, Helander et al. Citation2013). Their resurgence in ecosystems has inevitably influenced other species, including those of conservation concern, through trophic interactions (Kojola et al. Citation2009, O’Brien et al. Citation2018). Therefore, knowledge on the composition of apex predator diets may provide estimates on the magnitude of any negative effects on predated species of conservation concern.
Optimal diet theory predicts that, when the abundance of a preferred food type increases, the number of less preferred food types in the diet will shrink (Pyke et al. Citation1977). Many studies have demonstrated that generalist avian predators show spatiotemporal variations in dietary composition at different scales. Dietary composition may change over time due to the raptors responding to annual fluctuations in their preferred prey, requiring a switch to alternative prey (Korpimäki et al. Citation1990, Reif et al. Citation2001), or as a consequence of long-term changes in the availability of the most important prey and/or land-use patterns (Rutz & Bijlsma Citation2006, Hoy et al. Citation2017, Rebollo et al. Citation2017). Spatial variations in diet within a population may be the result of prey selection, which depends on individual predator traits (Rutz et al. Citation2006, Nadjafzadeh et al. Citation2016a), fine-scale habitat heterogeneity (Katzner et al. Citation2005, Zub et al. Citation2010) and variations in environment and/or prey availability among regions (Nadjafzadeh et al. Citation2016a, Clouet et al. Citation2017). On the other hand, a diet may change over time because a predator starts to exploit a broader environmental gradient as its population increases (Ekblad et al. Citation2016).
In the temperate and boreal regions of Europe, the White-tailed Eagle Haliaeetus albicilla is the largest avian predator associated with coastal and freshwater habitats, mainly feeding on fish and waterfowl, but also consuming mammals as well as terrestrial birds (Helander Citation1983, Sulkava et al. Citation1997, Struwe-Juhl Citation2003, Whitfield et al. Citation2013, Sándor et al. Citation2015, Nadjafzadeh et al. Citation2016b, Kamarauskaitė et al. Citation2020). The dietary composition of this eagle differs between different parts of its distribution range (e.g. Greenland & Germany: Nadjafzadeh et al. Citation2016a) and within the same region (Sweden: Helander Citation1983, Finland: Sulkava et al. Citation1997). European White-tailed Eagle populations started to recover after organochloride pesticides caused suppression in the mid-1980s and mid-1990s, and still continue to grow, with pairs establishing their breeding territories in new areas, or in less suitable sites in areas already occupied by conspecifics, resulting in higher habitat heterogeneity in the distribution of the species (Treinys et al. Citation2016, Heuck et al. Citation2017). Due to its association with this broader spectrum of habitats, the diet of the White-tailed Eagle has changed over several decades to reflect prey availability (Ekblad et al. Citation2016). Therefore, in any given population, a broadening of dietary niche over time may be expected.
The estimation of dietary composition based on prey remains collected from nests and under nests during the breeding season has several shortcomings (Valkama et al. Citation1999, Nadjafzadeh et al. Citation2016a). However, this method can still be useful as an index for evaluating spatial and temporal differences in prey composition (Ekblad et al. Citation2016), as well as providing insights into the relative frequencies of the predated species. In the present study, we determined the following: (1) the main groups of species found as prey items in successful nests during the breeding period; (2) the extent of predation on bird species of conservation concern during that period; and (3) variations in the main prey groups and dietary breadth through the years and along the environmental gradient.
We made the following predictions: (1) fish and water birds would be the most frequent taxa consumed during the breeding season, similarly to other areas of Europe; (2) predation events on bird species of conservation concern would be rare because the White-tailed Eagle relies on locally abundant fish and water bird species (also because bird species of conservation concern are usually rare compared to all available prey); (3) the frequency of prey groups and the dietary breadth would vary across different habitats because of the different availability of feeding habitats along the environmental gradient; (4) dietary breadth would be higher in pairs associated with inland habitats compared to coastal habitats because of the lower availability of typical feeding areas; and (5) the proportion of main prey species would decrease while the proportion of less typical prey species (i.e. terrestrial birds, mammals) and the dietary breadth would increase over time because the White-tailed Eagle population is continuing to grow; consequently, the accessibility to optimal feeding habitats and preferred prey would decrease.
Methods
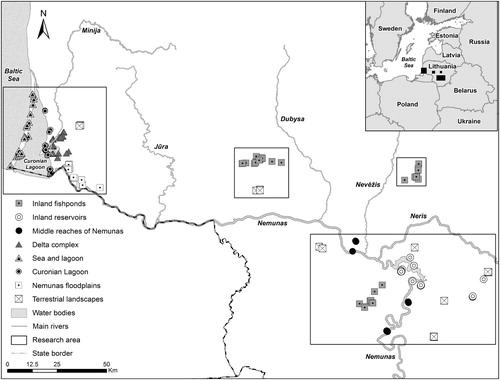
A search for White-tailed Eagle nesting territories was conducted in coastal and inland areas of Lithuania, located in Eastern Europe (), with observations being made of White-tailed Eagle behaviour (hunting, territoriality, food delivery), followed by searching for new, or monitoring already known, nest sites (field procedure previously described in more detail in Dementavičius Citation2007, Treinys et al. Citation2016, Dementavičius et al. Citation2019). Prey and their remains found in the nests were collected once per season during the ringing of nestlings in May–June between 1997 and 2018. Altogether, we observed 302 successful breeding attempts of 60 White-tailed Eagle pairs, which represents approximately one-third of the Lithuanian population. The prey and their remains were identified to the lowest identifiable taxonomic level. Fresh, whole or otherwise easily identifiable prey items were identified in the field. The remaining items (body parts, bones, feathers, fur, skulls, etc.) were collected and identified later, using reference animal collections in the T. Ivanauskas Zoological Museum (Kaunas, Lithuania). The ‘minimum approach’ (Milchev et al. Citation2012) was applied when counting the number of prey items from the remains (i.e. if two different parts of the same species were found, they were recorded as belonging to only one individual, unless they differed in size, indicating that they were from different individuals). In total, 2373 prey items, belonging to the Aves, Mammalia and Pisces classes, were found and 2272 items were identified to the lower taxonomic level.
Figure 1. Locations of sampled nest sites of the White-tailed Eagle and their assignment to the eight habitat categories in Lithuania.
To assess the White-tailed Eagle threat to species of conservation concern, we analysed the occurrence of individuals of the bird species preyed by this raptor (1) listed in Annex I of the EU Birds Directive (Directive 2009/147/EC) and (2) assigned to the International Union for Conservation of Nature (IUCN) critically endangered, endangered and vulnerable categories under the European assessment (IUCN Citation2019). Dietary niche breadth was computed using Levins’ index formula (B = 1/Σpi2, where B is the dietary niche breadth and pi is the proportion of the diet contributed by prey item i), which is commonly used in raptor studies (Sulkava et al. Citation1997, Katzner et al. Citation2005, Whitfield et al. Citation2009, Clouet et al. Citation2017). Seventy-one prey genera were used to calculate the index (for this analysis, we used ‘Anas’ to include individuals of Mareca, Anas and Spatula, and ‘Larus’ to include individuals of Hydrocoloeus, Chroicocephalus and Larus). To analyse the spatial and temporal dynamics of the dietary composition, all prey items were assigned to one of five groups, as follows: (1) Northern Pike Esox lucius (most abundant single prey); (2) other fish; (3) water birds (principally, Anseriformes, grebes, divers, gulls, herons, the Eurasian Coot Fulica atra, etc.); (4) terrestrial birds (waders, passerines, raptors, storks, the Common Crane Grus grus, etc.); and (5) mammals.
The breeding territories of the sampled White-tailed Eagle pairs were distributed in different areas and were all associated with diverse habitats. Thus, instead of using several continuous explanatory variables, we assigned the breeding territories to one of eight habitat categories that best represented the dominant foraging habitats, and also correspond to an environmental gradient: (1) sea and lagoon (8 pairs); (2) the Curonian Lagoon (8 pairs); (3) delta complex (11 pairs); (4) the Nemunas floodplains (7 pairs); (5) inland fishponds (10 pairs); (6) inland reservoirs (6 pairs); (7) the middle reaches of the Nemunas river (3 pairs); and (8) terrestrial landscape (7 pairs) (). For assignment of White-tailed Eagle breeding pairs to the habitat categories, we linked the location of nest sites to the dominant type of habitat within a 3.5 km (coastal area, 1–4 habitat categories) and 7 km (inland area, 5–8 habitat categories) radius around them. These distances were selected as representative of an area covering the most important foraging locations in the breeding territories, based on observed feeding flights during the breeding season in Lithuania (authors’ data) and values reported in the literature (range 2.5–15 km from a nest: references in Heuck et al. Citation2017). The two radii for the coastal and inland pairs were used because of different distances covered during observed hunting flights; also was considered the nearly two-fold difference in the breeding densities of White-tailed Eagles nesting in coastal compared to inland areas: in 2015, the mean nearest neighbour distance (that permit to obtain the minimum utilized home range, see Penteriani et al. Citation2001) was 3.6 km for pairs nesting in coastal areas and 7 km for inland pairs (author's data), suggesting differences in the extent of home-ranges.
We estimated the effect of habitat category on the occurrence of one out of five prey group items in successful nests (0 = absent, 1 = at least one item of a certain prey group), using a generalized linear mixed model (GLMM) with a binomial error structure and logit link function. The identity of the White-tailed Eagle breeding territory was included as a random factor. We compared the Akaike information criterion (AIC) corrected for a small sample size (AICc) and the weight of that model to the reduced model, which included only a random factor, but no fixed effect. To separate a good model from a less supported model, the threshold of ΔAICc ≤2 was used (Krüger et al. Citation2012). We tested annual variations in the occurrence of prey items belonging to (1) Northern Pike, (2) other fish, (3) water birds, (4) terrestrial birds and (5) mammal groups between 2005 and 2018, using generalized additive models (GAMs) (Jones & Wrigley Citation1995). GAMs were fitted applying binomial family, logit link, smooth function for the explanatory variable ‘year’ was limited to 5 (k = 5) (Heinänen et al. Citation2017). Data collected before 2005 were excluded because these represented small and fragmented annual samples; only data collected from 277 successful nests in 2005–2018 were used for this analysis. The packages lme4 (Bates et al. Citation2015), MuMIn (Bartoń Citation2019) and mgcv (Wood Citation2011) were used in the statistical environment R version 3.6.0 (R Core Team Citation2019). We reported marginal and conditional R2 estimated by a ‘theoretical’ method applied to binomial family models (Nakagawa et al. Citation2017).
Results
Dietary composition
During the 1997–2018 period, fish were the most common prey, by number, comprising 62% of the sampled prey individuals, with 24% of the prey items being water birds, 10% terrestrial birds and 4% mammals (). The Northern Pike was the most abundant single species, constituting 19% of all the identified prey (n = 2272) and being detected in 47% of the nests (n = 302). The second most abundant fish species was Common Bream Abramis brama (10%, n = 2272). Eurasian Coot (7%, n = 2272) and Mallard Anas platyrhynchos (4%, n = 2272) were the most frequent bird species found as prey items in the White-tailed Eagle nests.
Table 1. List of prey items found in the nests of White-tailed Eagles. % given from the number of prey identified (n = 2272).
Spatial and temporal variations in diet in 2005–2018
The proportions of the main prey groups strongly varied among the samples collected from nests located in different habitats (χ2 = 210, df = 28, P < 0.0001), with a general pattern of a decreasing share of fish and an increasing share of birds being observed from coastal to terrestrial habitats (). The occurrence of prey items belonging to one of four prey groups (i.e. Northern Pike, other fish, terrestrial birds, mammals) varied significantly across pairs breeding in different habitats (). The occurrence of predated water birds in the nests, however, differed the least among the habitat categories, as indicated by the model with habitat category as a fixed effect being 1.8-fold less supported compared to the null model. The occurrence of Northern Pike, water birds and mammal prey items in the nests could also be explained by the habitat category together with the breeding territory identity (cf. marginal and conditional R2 in the corresponding models; ). Breeding territory identity, however, contributed only marginally to explaining the occurrence of terrestrial bird and other fish items in the nests located in different habitats.
Figure 2. Levins’ index of dietary niche breadth and proportions of five prey groups in samples collected from the White-tailed Eagle nests associated with eight habitat categories.
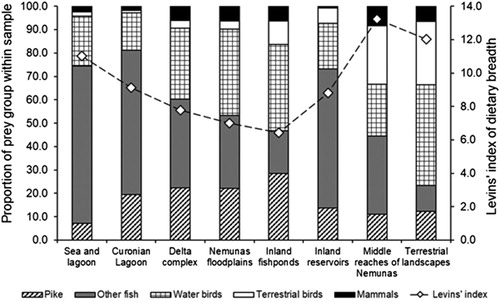
Table 2. Summary of generalized linear mixed models analysing the variation in occurrence of prey belonging to one of the five groups among habitat categories (inland fishponds – reference habitat group in models). R2m – proportion of variance explained by fixed variable (i.e. habitat category), R2c – proportion of variance explained by fixed (i.e. habitat category) and random (i.e. breeding territory identity) variables. * – significant difference at P < 0.05. Δ – difference between AICc of model including habitat category (fixed) and breeding territory identity (random) variables and corresponding model including only random variable.
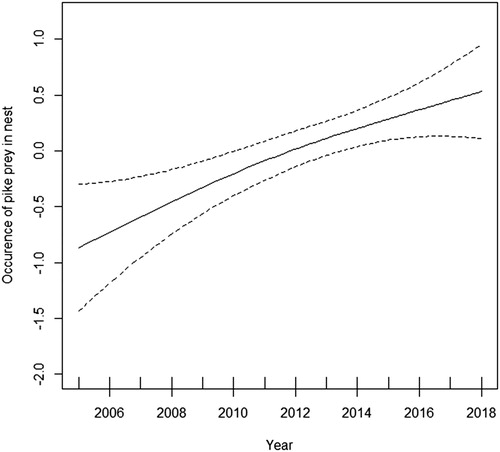
The occurrence of Northern Pike in the nests of the White-tailed Eagle significantly increased throughout the period of the study (GAM: year effect P < 0.01; ). The occurrence of prey items belonging to other fish, water birds and terrestrial birds, however, did not vary significantly over the study period (all P > 0.4 for year effect in univariate GAMs).
Dietary niche breadth and its spatial and temporal variation in 2005–2018
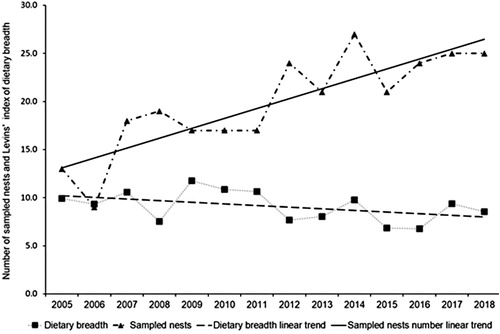
Levins’ index of White-tailed Eagle dietary niche breadth was 11.7. It varied markedly across habitat categories, the smallest value of 6.4 indicating the least diverse diet, observed in pairs associated with inland fishponds, while the highest value of 13.2 indicated the most diverse diet in pairs breeding in the middle reaches of the Nemunas river (). The overall pattern, however, was not one of a gradual increase in dietary niche breadth along the environmental gradient, from areas with the highest availability of aquatic habitats to the areas dominated by terrestrial habitats. The annual number of sampled successful nests increased (r = 0.85, P < 0.001, n = 14), indicating population growth, but Levins’ index of dietary niche breadth did not increase between 2005 and 2018 (r = –0.45, P > 0.1, n = 14; ).
Predation on protected bird species
The White-tailed Eagles preyed upon 57 bird species during the breeding period between 1997 and 2018, seven of which are included in Annex I of the EU Birds Directive (). Individuals belonging to protected bird species comprised approximately 3% (i.e. 21 individuals) of the avian prey (n = 772) and approximately 1% of all identified prey (n = 2272). Two species – represented by 14 preyed individuals – out of seven species included in Annex I are abundant in Lithuania; that is, the Common Crane and White Stork Ciconia ciconia. Individuals of other protected bird species occurred in the nests only occasionally. Altogether, 20 individuals, belonging to one endangered and six vulnerable bird species in Europe, were recorded. Again, most of these individuals (11 out of 20) belonged to one species, the Eurasian Lapwing Vanellus vanellus.
Discussion
The most common prey items were fish, followed by water birds, which together accounted for 86% of the prey remains found in the nests of White-tailed Eagles during the breeding season in 1997–2018. The frequency of the prey groups varied along the environmental gradient, with a general pattern emerging of increasing avian prey in pairs breeding in areas with less abundant aquatic habitats, although the dietary niche breadth index did not show such a trend. Contrary to our expectation, alternative prey frequency (i.e. terrestrial birds, mammals) and dietary niche breadth did not increase during the study period. Instead, the occurrence of Northern Pike, which was the most frequent single prey species, increased throughout the study period. Individuals of bird species of conservation concern constituted only a small fraction of all the prey identified, suggesting low conflict of the White-tailed Eagle with different conservation aims for rare bird species, at least in the region east of the Baltic Sea.
Diet
Fish were the main prey items among the recorded prey remains at 62%, which is similar to the mean values for White-tailed Eagle breeding season diets elsewhere in Europe: Finland – 42% (Sulkava et al. Citation1997); Romania – 45% (Sándor et al. Citation2015); Belarus – 53% (Ivanovski Citation2012); Swedish Lapland and the Baltic coast – 53% and 60%, respectively (Helander Citation1983); and Germany – 73% (Struwe-Juhl Citation2003). On the other hand, fish remains have rarely been found in Scotland (Whitfield et al. Citation2013) and in East Siberia, at Lake Baikal (Mlíkovksý Citation2009), where avian prey comprise the largest proportion of the White-tailed Eagle diet. The Northern Pike (19%) was the most common prey species found by us, which is similar to other White-tailed Eagle diets in northern European countries (Helander Citation1983, Sulkava et al. Citation1997) and Belarus (Ivanovski Citation2012). Eurasian Coot (7%) and Mallard (4%) were the most frequent avian prey items, similarly to the frequencies found in the breeding White-tailed Eagle diets in Belarus (Ivanovski Citation2012). Mallard also accounted for approximately 4% of the White-tailed Eagle breeding-season diet in northern Europe (Helander Citation1983, Sulkava et al. Citation1997), although the Eurasian Coot has been more frequently observed among prey items in Germany (Struwe-Juhl Citation2003) and Romania (Sándor et al. Citation2015). The White-tailed Eagle diet in Lithuania may be characterized as transitional between European countries located to the north, east and west. Variations in the diet of a well-known generalist predator, the Golden Eagle Aquila chrysaetos, among regions are also evident in the western United States (Bedrosian et al. Citation2017) and in the Pyrenees (Clouet et al. Citation2017). Similarly as suggested by Schweiger et al. (Citation2015), it is likely that the local diet of a generalist predator is defined by the availability of foraging habitat types, determined by geographical location (Nadjafzadeh et al. Citation2016a) and mediated by the availability of optimal-sized prey species (i.e. 0.5–1 kg for the White-tailed Eagle, Sulkava et al. Citation1997).
Spatial dietary variation
The highest frequency of fish prey was found in White-tailed Eagle pairs breeding close to the Baltic Sea and the Curonian Lagoon, and the lowest in pairs breeding near the middle reaches of the Nemunas river and in terrestrial landscapes. Pairs breeding near large inland reservoirs, however, showed a similarly high proportion of fish prey to those breeding in the coastal area. The proportion of water birds was the most consistent across habitat types compared to other prey categories. Spatial differences in the White-tailed Eagle diet were repeatedly observed, not only between different regions, but also within the same region at the fine habitat gradient scale (Helander Citation1983, Sulkava et al. Citation1997, Ekblad et al. Citation2016). In our study, the proportion of avian prey was highest in pairs associated with more terrestrial landscapes, contrary to the relationship reported for the Åland Islands archipelago, where the proportion of birds in the diet decreased when the proportion of land in the nesting territories increased (Ekblad et al. Citation2016). Moreover, the occurrence of other fish species and terrestrial birds among the prey in our study was explained by habitat, while the occurrence of Northern Pike, water birds and mammals were additionally explained by breeding territory identity. The breeding territory effect may be caused by the specialization of the White-tailed Eagle individuals to hunt different prey and/or inter-territory heterogeneity in that food type. Foraging behaviour is a hierarchical process that covers selection of foraging patch, the search for and identification of suitable prey items and, finally, capture of the prey (Rutz et al. Citation2006). Individuals of a generalist predator in the population may specialize in different prey (Katzner et al. Citation2005, Whitfield et al. Citation2009), and foraging differences among individual White-tailed Eagles in the same population have been observed (Nadjafzadeh et al. Citation2016a). Most likely, however, the breeding territory effect on the occurrence of certain prey in our study may be an outcome of different prey availability, but not of different preferences among individuals. According to prediction by optimal foraging theory, food types are first ranked by the ratio of the food value to its handling time (Pyke et al. Citation1977), thus the breeding territory effect on the consumption of certain prey is plausibly an outcome of White-tailed Eagle selective behaviour, based on the diversity/structure of the prey species and their availabilities in the home-ranges, irrespective of individual birds specializing in certain types (see also the Prey Availability Hypothesis: Whitfield et al. Citation2009).
A maximum of approximately two-fold difference in dietary niche breadth in White-tailed Eagle pairs breeding in different habitats was found. An approximately two- to three-fold spatial difference in degree of dietary specialization has been commonly reported in other avian predator studies: in the Finnish White-tailed Eagle population (Sulkava et al. Citation1997); in two Golden Eagle populations inhabiting opposite sides of the Pyrenees (Clouet et al. Citation2017); and in local populations of Imperial Eagle Aquila heliaca in Kazakhstan (Katzner et al. Citation2005). Whitfield et al. (Citation2009) found, however, that Golden Eagle dietary niche breadths in the northwest islands of Scotland were similar (range of Levins’ index 3.7–4.8), despite differences in the prey species consumed. Furthermore, the least diverse diet in our study was observed in White-tailed Eagle pairs associated with inland fishponds. The aquaculture areas support a much higher density of only a few fish species compared to the densities and fish species diversity in natural water bodies (Tuvi & Väli Citation2007). Thus, fishponds may act similarly to a colonial prey source, decreasing the dietary diversity of avian predators nesting nearby (Katzner et al. Citation2005). Just as expected, the most diverse diet was found in terrestrial-dominant areas because such breeding territories lack large water bodies, and so the prey is more diverse, including terrestrial birds, compensating for the limited availability of a staple food resource (i.e. behaviour according to the Alternative Prey Hypothesis: Reif et al. Citation2001).
Temporal dietary variation
Predation on terrestrial birds did not increase as initially expected, despite the White-tailed Eagle population growing over the course of the study period. The occurrence of Northern Pike among the prey remains, however, did increase significantly. The White-tailed Eagle prefers fish over avian prey (Nadjafzadeh et al. Citation2016b), and the Northern Pike is its principal prey among fish (see above). However, it is not likely that this increase in the White-tailed Eagle diet can be explained by a behavioural shift from another prey to pike over the study period (in the case of constant pike availability). The expansion in environmental gradient due to the establishment of new White-tailed Eagle territories in areas with a greater abundance of Northern Pike in the foraging areas is also not likely to explain the observed pattern; White-tailed Eagle pairs firstly settled in coastal habitats and near inland fishponds, only later establishing breeding territories in natural inland areas during the population recovery since the mid-1990s (Treinys et al. Citation2016). However, the proportion of Northern Pike in prey remains was higher in pairs nesting in coastal areas (except for pairs nesting on the Curonian Spit) and in pairs associated with inland fishponds (20–29%) compared to pairs nesting in other inland habitats (11–14%). We explain the increased proportion of pike in the White-tailed Eagle diet as a numerical response by the eagles to the increased availability of this prey species in the water bodies. In Lithuania, since around 2005, the annual stocking of Northern Pike in state-owned water bodies has increased two- to three-fold compared to preceding years (Virbickas et al. in prep.). Of note, the increased availability of the preferred prey in water bodies on a country scale probably best explains not only why pike were being consumed more frequently as time went on, but also why the dietary niche breadth and the proportion of alternative prey in the diet did not increase with the increase of the White-tailed Eagle population. The proportion of pike declined in the prey material collected from White-tailed Eagle nests between 1985 and 2010 in the Åland Islands archipelago (Finland); this was consistent with a decrease in pike in the Baltic Sea (Ekblad et al. Citation2016). In summary, we suggest that the White-tailed Eagle has not become food-limited, despite its population nearly doubling between 2005 and 2018 in our study area, as well as in Lithuania as a whole (from approximately 90 pairs in 2005 [Treinys et al. Citation2016] to approximately 160 pairs in 2018 [Dementavičius unpubl.]).
Implications
In the present study, we found that, during its breeding season, the White-tailed Eagle harvests only solitary individuals of bird species of conservation concern. Of course, the method used to study its diet in the present study has its limitations, as has been shown by video-recording analysis (Lewis et al. Citation2004, Selås et al. Citation2007), and it is also known that prey with soft bones (e.g. nestlings) may not be found in White-tailed Eagle nests (Ekblad et al. Citation2016). Lourneço et al. (Citation2011) did not find any effect of the total number of prey on the percentage of rare prey (i.e. mesopredators) within the diet samples of top predators. Therefore, it is unlikely that the method used in this study considerably underestimates the proportion of bird species of conservation concern. Most of these bird species could be described as ‘marginal alternative prey’ of the White-tailed Eagle, being locally scarce compared to the preferred fish and frequently taken, locally abundant water birds (see above). Thus, it could be suggested that the recovered White-tailed Eagle population, which has expanded into a broad spectrum of local habitats, does not limit populations of bird species of conservation concern, at least not by the direct removal of individuals during the breeding season.
It is necessary to emphasize, however, that this suggestion should not be applied directly to other regions because: (1) the diets of generalist predators correspond to the regional diversity and abundance of the principal prey species, mediated by geographical location; (2) within a region, their diet varies along environmental gradients, and the relationship between habitat and prey category abundance in the diet may be region-specific; (3) their diet may be shaped by foraging habitat conditions at the breeding-territory scale; and (4) the local short- and long-term dynamics of primary-prey abundances may trigger temporal changes in alternative prey selection. Hence, we encourage the use of caution when estimating/forecasting the effect of the White-tailed Eagle – or any other generalist predator – on potential prey species of conservation concern in one area, based on findings from other regions and/or older studies. Video-recording of provisioning events at nests, combined with conventional methods to assess diet, would be the best solution for estimating the predation on species of conservation concern, at least during the breeding season.
Acknowledgments
We are thankful for the anonymous Reviewer for valuable suggestions to improve the manuscript.
References
- Bartoń, K. 2019. MuMIn: multi-model inference. R package version 1.43.6. https://CRAN.R-project.org/package=MuMIn.
- Bates, D., Maechler, M., Bolker, B. & Walker, S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48. doi: 10.18637/jss.v067.i01
- Bedrosian, G., Watson, J.W., Steenhof, K., Kochert, M.N., Preston, C.R., Woodbridge, B., Williams, G.E., Keller, K.R. & Crandall, R.H. 2017. Spatial and temporal patterns in Golden Eagle diets in the Western United States, with implications for conservation planning. J. Raptor Res. 51: 347–367. doi: 10.3356/JRR-16-38.1
- Chakarov, N. & Krüger, O. 2010. Mesopredator release by an emergent superpredator: a natural experiment of predation in a three level guild. PLOS One 5: e15229. doi: 10.1371/journal.pone.0015229
- Clouet, M., Gerard, J.F., Goar, J.L., Goulard, M., González, L., Rebours, I. & Faure, C. 2017. Diet and breeding performance of the Golden Eagle Aquila chrysaetos at the eastern and western extremities of the Pyrenees: an example of intra-population variability. Ardeola 64: 347–361. doi: 10.13157/arla.64.2.2017.ra4
- Delibes-Mateos, M., Redpath, S.M., Angulo, E., Ferreras, P. & Villafuerte, R. 2007. Rabbits as a keystone species in southern Europe. Biol. Conserv. 137: 149–156. doi: 10.1016/j.biocon.2007.01.024
- Dementavičius, D. 2007. White-tailed Eagle (Haliaeetus albicilla) in Lithuania: population numbers and trends 1900–2007. Acta Zool. Litu. 17: 281–285. doi: 10.1080/13921657.2007.10512845
- Dementavičius, D., Rumbutis, S., Vaitkuvienė, D., Dagys, M. & Treinys, R. 2019. No adverse effects on Lesser Spotted Eagle breeding in an area of high White-tailed Eagle density. J. Ornithol. 160: 453–461. doi: 10.1007/s10336-019-01625-2
- Ekblad, C.M.S., Sulkava, S., Stjernberg, T.G. & Laaksonen, T.K. 2016. Landscape-scale gradients and temporal changes in the prey species of the White-tailed Eagle (Haliaeetus albicilla). Ann. Zool. Fenn. 53: 228–240. doi: 10.5735/086.053.0401
- Fasce, P., Fasce, L., Villers, A., Bergese, F. & Bretagnolle, V. 2011. Long-term breeding demography and density dependence in an increasing population of Golden Eagles Aquila chrysaetos. Ibis 153: 581–591. doi: 10.1111/j.1474-919X.2011.01125.x
- Heinänen, S., Žydelis, R., Dorsch, M., Nehls, G. & Skov, H. 2017. High-resolution sea duck distribution modeling: relating aerial and ship survey data to food resources, anthropogenic pressures, and topographic variables. Condor 119: 175–190. doi: 10.1650/CONDOR-16-57.1
- Helander, B. 1983. Reproduction of the white-tailed sea eagle Haliaeetus albicilla (L.) in Sweden, in relation to food and residue levels of organochlorine and mercury compounds in the eggs. PhD Thesis, University of Stockholm, Sweden.
- Helander, B., Bignert, A., Herrmann, C. & Stjernberg, T. 2013. White-tailed Eagle productivity. HELCOM core indicator report. http://helcom.fi/Core%20Indicators/HELCOM-CoreIndicator-Whitetail_Eagle_productivity.pdf. Accessed 20 December 2014.
- Heuck, C., Herrmann, C., Schabo, D.G., Brandl, R. & Albrecht, J. 2017. Density-dependent effects on reproductive performance in a recovering population of White-tailed Eagles Haliaeetus albicilla. Ibis 159: 297–310. doi: 10.1111/ibi.12444
- Hoy, S.R., Petty, S.J., Millon, A., Whitfield, D.P., Marquiss, M., Anderson, D.I.K., Davison, M. & Lambin, X. 2017. Density-dependent increase in superpredation linked to food limitation in a recovering population of northern goshawks Accipiter gentilis. J. Avian Biol. 48: 1205–1215. doi: 10.1111/jav.01387
- IUCN. 2019. The IUCN red list of threatened species. Version 2019-3. http://www.iucnredlist.org. Downloaded on 10 December 2019.
- Ivanovski, V.V. 2012. Birds of Prey in Belorussian Poozerie. Vitebsk State University, Vitebsk (in Russian).
- Kamarauskaitė, A., Dementavičius, D., Skuja, S., Dagys, M. & Treinys, R. 2020. Interaction between the White-tailed Eagle and Common Buzzard estimated by diet analysis and brood defence behaviour. Ornis Fenn. 97: 26–37.
- Katzner, T.E., Bragin, E.A., Knick, S.T. & Smith, A.T. 2005. Relationship between demographics and diet specificity of Imperial Eagles Aquila heliaca in Kazakhstan. Ibis 147: 576–586. doi: 10.1111/j.1474-919X.2005.00443.x
- Jones, K. & Wrigley, N. 1995. Generalized additive models, graphical diagnostics, and logistic regression. Geogr. Anal. 27: 1–21. doi: 10.1111/j.1538-4632.1995.tb00333.x
- Kojola, I., Tuomivaara, J., Heikkinen, S., Heikura, K., Kilpeläinen, K., Keränen, J., Paasivaara, A. & Ruusila, V. 2009. European wild forest reindeer and wolves: endangered prey and predators. Ann. Zool. Fenn. 46: 416–422. doi: 10.5735/086.046.0602
- Korpimäki, E., Huhtala, K. & Sulkava, S. 1990. Does the year-to-year variation in the diet of eagle and Ural owls support the alternative prey hypothesis? Oikos 58: 47–54. doi: 10.2307/3565359
- Krüger, O., Chakarov, N., Nielsen, J.T., Volkher, L., Grünkorn, T., Struwe-Juhl, B. & Møller, A.P. 2012. Population regulation by habitat heterogeneity or individual adjustment? J. Anim. Ecol. 81: 330–340. doi: 10.1111/j.1365-2656.2011.01904.x
- Lewis, S.B., Fuller, M.R. & Titus, K. 2004. A comparison of 3 methods for assessing raptor diet during the breeding season. Wildl. Soc. Bull. 32: 373–386. doi: 10.2193/0091-7648(2004)32[373:ACOMFA]2.0.CO;2
- Lourenço, R., Santos, S.M., Rabaça, J.E. & Penteriani, V. 2011. Superpredation patterns in four large European raptors. Popul. Ecol. 53: 175–185. doi: 10.1007/s10144-010-0199-4
- Milchev, B., Spassov, N. & Popov, V. 2012. Diet of the Egyptian vulture (Neophron percnopterus) after livestock reduction in Eastern Bulgaria. North West J. Zool. 8: 315–323.
- Mlíkovský, J. 2009. The food of the White-tailed Sea Eagle (Haliaeetus albicilla) at Lake Baikal, East Siberia. Slovak. Raptor J. 3: 35–39. doi: 10.2478/v10262-012-0031-5
- Nadjafzadeh, M., Hofer, H. & Krone, O. 2015. Lead exposure and food processing in white-tailed eagles and other scavengers: an experimental approach to simulate lead uptake at shot mammalian carcasses. Eur. J. Wildl. Res. 61: 763–774. doi: 10.1007/s10344-015-0953-1
- Nadjafzadeh, M., Hofer, H. & Krone, O. 2016b. Sit-and-wait for large prey: foraging strategy and prey choice of White-tailed Eagles. J. Ornithol. 157: 165–178. doi: 10.1007/s10336-015-1264-8
- Nadjafzadeh, M., Voigt, C.C. & Krone, O. 2016a. Spatial, seasonal and individual variation in the diet of White-tailed Eagles Haliaeetus albicilla assessed using stable isotope ratios. Ibis 158: 1–15. doi: 10.1111/ibi.12311
- Nakagawa, S., Johnson, P.C.D. & Schielzeth, H. 2017. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14: 20170213. doi: 10.1098/rsif.2017.0213
- Newton, I. 1980. The role of food in limiting bird numbers. Ardea 68: 11–30.
- Newton, I. 2003. The role of natural factors in the limitation of bird of prey numbers: a brief review of the evidence. In Thompson, D.B.A., Redpath, S.M., Fielding, A.H., Marquiss, M. & Galbraith, C.A. (eds) Birds of Prey in a Changing Environment, 5–23. Scottish Natural Heritage/The Stationery Office, Edinburgh.
- O’Brien, T.G., Kinnaird, M.F., Ekwanga, S., Wilmers, C., Williams, T., Oriol-Cotterill, A., Rubenstein, D. & Frank, L.G. 2018. Resolving a conservation dilemma: vulnerable lions eating endangered zebras. PLOS One 13: e0201983. doi: 10.1371/journal.pone.0201983
- Penteriani, V., Faivre, B. & Frochot, B. 2001. An approach to identify factors and levels of nesting habitat selection: a cross-scale analysis of Goshawk preferences. Ornis Fenn. 78: 159–167.
- Preston, C.R., Jones, R.E. & Horton, N.S. 2017. Golden Eagle diet breadth and reproduction in relation to fluctuations in primary prey abundance in Wyoming’s Bighorn Basin. J. Raptor Res. 51: 334–346. doi: 10.3356/JRR-16-39.1
- Pyke, G.H., Pulliam, H.R. & Charnov, E.L. 1977. Optimal foraging: a selective review of theory and tests. Q. Rev. Biol. 52: 137–154. doi: 10.1086/409852
- R Core Team. 2019. R version 3.6.0. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
- Rebollo, S., Martínez-Hesterkamp, S., García-Salgado, G., Pérez-Camacho, L., Fernández-Pereira, J.M. & Jenness, J. 2017. Spatial relationships and mechanisms of coexistence between dominant and subordinate top predators. J. Avian. Biol. 48: 1226–1237. doi: 10.1111/jav.01337
- Reif, V., Tornberg, R., Jungell, S. & Korpimäki, E. 2001. Diet variation of common buzzards in Finland supports the alternative prey hypothesis. Ecography 24: 267–274. doi: 10.1034/j.1600-0587.2001.240304.x
- Rutz, C. & Bijlsma, R.G. 2006. Food-limitation in a generalist predator. Proc. R. Soc. B: Biol. Sci. 273: 2069–2076. doi: 10.1098/rspb.2006.3507
- Rutz, C., Whittingham, M.J. & Newton, I.P. 2006. Age-dependent diet choice in an avian top predator. Proc. R. Soc. B: Biol. Sci. 273: 579–586. doi: 10.1098/rspb.2005.3353
- Sándor, A., Alexe, V., Marinov, M., Doroşencu, A., Domşa, C. & Kiss, B.J. 2015. Nest-site selection, breeding success, and diet of white-tailed eagles (Haliaeetus albicilla) in the Danube Delta, Romania. Turk. J. Zool. 39: 300–307. doi: 10.3906/zoo-1401-64
- Schweiger, A., Fünfstück, H.J. & Beierkuhnlein, C. 2015. Availability of optimal-sized prey affects global distribution patterns of the golden eagle Aquila chrysaetos. J. Avian Biol. 46: 81–88. doi: 10.1111/jav.00396
- Selås, V., Tveiten, R. & Aanonsen, O.M. 2007. Diet of Common Buzzards (Buteo buteo) in southern Norway determined from prey remains and video recordings. Ornis Fenn. 84: 97–104.
- Struwe-Juhl, B. 2003. Why do White-tailed Eagles prefer coots? In Helander, B., Marquiss, M. & Bowerman, B. (eds) Sea Eagle 2000, 317–325. Swedish Society for Nature Conservation (SNF), Stockholm.
- Sulkava, S., Tornberg, R. & Koivusaari, J. 1997. Diet of the white-tailed eagle Haliaeetus albicilla in Finland. Ornis Fenn. 74: 65–78.
- Treinys, R., Dementavičius, D., Rumbutis, S., Švažas, S., Butkauskas, D., Sruoga, A. & Dagys, M. 2016. Settlement, habitat preference, reproduction, and genetic diversity in recovering the white-tailed eagle Haliaeetus albicilla population. J. Ornithol. 157: 311–323. doi: 10.1007/s10336-015-1280-8
- Tuvi, J. & Väli, Ü. 2007. The impact of the White-tailed Eagle Haliaeetus albicilla and the Osprey Pandion haliaetus on Estonian Common Carp Cyprinus carpio production: how large is the economic loss? Proc. Est. Acad. Sci. Biol. Ecol. 56: 209–223.
- Valkama, J., Korpimäki, E., Arroyo, B., Beje, P., Bretagnolle, V., Bro, E., Kenward, R.E., Manosa, S., Redpath, S.M., Thirgood, S. & Vinuela, J. 1999. Birds of prey as limiting factors of gamebird populations in Europe: a review. Biol. Rev. 80: 171–203. doi: 10.1017/S146479310400658X
- Whitfield, D.P., Marquiss, M., Reid, R., Grant, J., Tingay, R. & Evans, R.J. 2013. Breeding season diets of sympatric White-tailed Eagles and Golden Eagles in Scotland: no evidence for competitive effects. Bird Study 60: 67–76. doi: 10.1080/00063657.2012.742997
- Whitfield, D.P., Reid, R., Haworth, P.F., Madders, M., Marquiss, M., Tingay, R. & Fielding, A.H. 2009. Diet specificity is not associated with increased reproductive performance of Golden Eagles Aquila chrysaetos in Western Scotland. Ibis 151: 255–264. doi: 10.1111/j.1474-919X.2009.00924.x
- Wood, S.N. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 73: 3–36. doi: 10.1111/j.1467-9868.2010.00749.x
- Zub, K., Pugacewicz, E. & Jędrzejewska, B. 2010. Factors affecting habitat selection by breeding Lesser Spotted Eagles Aquila pomarina in Northeastern Poland. Acta Ornithol. 45: 105–114. doi: 10.3161/000164510X516155