?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this work, we use a boundary layer resolved model to improve a Lagrangian point particle model to simulate the combustion of single iron particles. By resolving the full boundary layer, mass and heat transfer are accurately modeled, including Stefan flow. Therefore, the model is suitable to improve point particle models. This work focuses on the first stage of iron combustion, which lasts up to the maximum temperature. Temperature- and composition-dependent properties are used and phase transitions from solid to liquid and liquid to gas are taken into account. The Nusselt and Sherwood correlations are investigated in conditions typical for iron particle combustion. It is found that the -film temperature is the best film rule to use to model heat- and mass transfer for iron particle combustion. The boundary layer resolved model is used to validate the point particle models. Then, the model is systematically elaborated by including a temperature-dependent particle density, slip velocity and Stefan flow. The individual and combined effect of these phenomena on the burn duration are investigated. Including all these effects decreases the time to maximum temperature by around
. Furthermore, it is shown that if one neglects physical phenomena like slip and Stefan flow, but uses the
-film rule instead of the
-film rule, errors cancel and still reasonable agreement is obtained with experiments.
Introduction
Micron-sized iron is considered as a promising metal fuel since it is inherently carbon-free, recyclable, compact and also cheap and widely available (Bergthorson et al. Citation2015). In turn, the iron-oxide particles can be captured and reduced back into iron particles using renewable energy sources, thereby enabling a carbon-free energy cycle.
For the development of practical iron fuel burning setups, it is needed to get a better understanding of the fundamental characteristics of iron combustion. Multiple experimental studies (Poletaev and Khlebnikova Citation2020; Sun, Dobashi, Hirano Citation2000; Tang et al. Citation2009; Wright, Higgins, Goroshin Citation2016) have contributed to the understanding of iron dust combustion. Ning et al. (Citation2021a, Citation2021b, Citation2022) performed single particle combustion experiments where the particles are ignited by a laser. The combustion process was divided into two stages, where the first stage lasts up to the maximum temperature. The burning process duration of the first stage is sensitive to the oxygen concentration and that the maximum particle temperature is a function of the oxygen concentration, saturating at elevated oxygen concentrations. The second stage, after the peak temperature, has a negligible effect on oxygen concentration. In this last stage of combustion, an intensity jump was observed, which was also observed during the combustion of other metal particles (Dreizin, Suslov, Trunov Citation1993). Li et al. (Citation2022) investigated the ignition and combustion process of single micron-sized iron particles in the hot gas flow of burned methane-oxygen-nitrogen mixture, and draw similar conclusions as Ning et al. (Citation2021a).
In our previous work (Thijs et al. Citation2022), a boundary-layer resolved transient model for iron particle combustion was presented. By resolving the full boundary layer, mass and heat transfer are accurately modeled, including Stefan flow. However, the development of accurate point particle models is of importance to simulate industrial burners with many particles, where the boundary layer around each particle cannot be resolved. The development of point particle models gained more attention in the last couple of years. Soo et al. (Citation2018) developed a numerical model for the combustion of a single metal particle, which was extended by Hazenberg and van Oijen (Citation2021) by taking into account more realistic parameters and combustion products were not assumed to vanish. Schiemann, Fischer, and Bergthorson (Citation2017) developed a numerical model which assumes the oxidation from to
, but used a predefined maximum temperature based on the dissociation temperature of
. Mi, Fujinawa, and Bergthorson (Citation2022) used solid-phase oxidation kinetics described by a parabolic rate to improve their iron combustion model in the solid phase, but they only focused on the ignition phase. The above described models make assumptions on the effect of slip, Stefan flow, or evaporation.
To enable modeling of single particle combustion without resolving the full boundary layers, reliable heat and mass transfer coefficients must be used. To model the heat transfer in the boundary layer, a common correlation used for the Nusselt number was proposed by Ranz and Marshall (Citation1952)
This correlation has been obtained by measuring the temperature of water droplets in an air atmosphere for ambient temperatures and is valid up to . The maximum temperature difference tested between the water droplet and the air stream was
and provides therefore a small variation in the thermophysical properties of air across the boundary layer.
Richter and Nikrityuk (Citation2012) performed numerical calculations of heat and fluid flow past spherical particles and non-spherical particles of various shapes. They developed a new correlation which is dependent on the sphericity and is valid from
up to
. For a spherical particle,
, their correlation becomes
They considered a temperature difference between the particles and fluid of , but used all constant material properties. Note that this correlation does not approach the theoretical limit of
if
.
The reference temperatures which are used to determine the transport properties differ in the literature. Ranz and Marshall (Citation1952) determined their transport properties with the -film rule, which is defined as the average between the far-field temperature and the particle temperature. Whitaker (Citation1972) used the gas temperature as a reference temperature, while Hubbard, Denny, and Mills (Citation1975) stated that for engineering calculations, the most appropriate reference state for the evaluation of properties is the
rule. The latter is also done in the earlier work regarding metal fuels (Hazenberg and van Oijen Citation2021; Mi, Fujinawa, Bergthorson Citation2022; Ravi, de Goey, van Oijen Citation2022).
Several authors (Fiszdon Citation1979; Whitaker Citation1972) investigated the effect of large temperature differences on the Nusselt correlations. Ellendt et al. (Citation2018) concluded that the Ranz-Marshall correlation can be applied to high-temperature differences in the laminar regime, with the properties calculated using the -film rule. They performed numerical simulations with Comsol Multiphysics 5.2, but did not show any results regarding their derived Nusselt numbers as a function of the Reynolds number. Instead, they only stated that their data fit the well-established Ranz and Marshall correlation very well (
). More recently, Jayawickrama et al. (Citation2021) investigated the Nusselt number with Stefan flow under large temperature differences. They proposed to use a volume averaged temperature, dependent on the Stefan velocity, Reynolds number and temperature.
In this work, we will use our boundary-layer resolved model (Thijs et al. Citation2022) to further improve the point particle models for the first stage of iron particle combustion. The Nusselt and Sherwood correlations are reviewed in flame-like conditions and the choice of reference temperature on the Nusselt correlations is investigated. Then, the point particle model is compared to the boundary-layer resolved model and the latter is used to investigate the effect on the time to maximum temperature when neglecting a temperature-dependent density, slip or Stefan flow.
The paper is organized as follows. Section 2 describes the methodology of the boundary-layer resolved model and the Lagrangian point particle model. Section 3 presents the results and discusses the above-mentioned points. Finally, conclusions are provided in Section 4.
Methodology
In this section, the methodology for simulating the combustion of single iron particles is described. First, the boundary layer resolved model is introduced, which is used as a validation tool for the Lagrangian point particle model.
Boundary layer resolved model
shows the computational domain used for the boundary layer resolved model. A 2D axi-symmetric model for the combustion of a single iron particle with diameter was developed, of which more details can be found in Thijs et al. (Citation2022).
Figure 1. Boundary layer resolved geometry (Thijs et al. Citation2022).
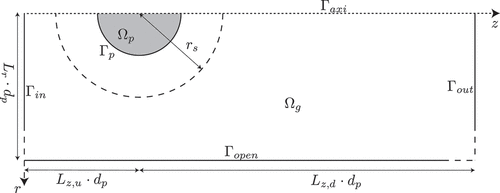
For the boundary layer resolved model the continuum approach will be used for modeling the mass and heat transfer. The governing equations for mass, species, momentum and energy in the gas phase read:
Here, ,
,
,
,
,
and
are the density, specific heat capacity at constant pressure, temperature, thermal conductivity, dynamic viscosity, velocity and pressure of the gas phase, respectively. The equations are solved on a body-fitted mesh with the “Comsol Multiphysics 5.5” package.
While in Thijs et al. (Citation2022) a kinetic model was used in the gas phase, this is not considered in this work. Therefore, only the species ,
,
and
are considered for the gas phase. The density of the gas mixture is based on the ideal gas law, the transport properties are based on kinetic gas theory and thermodynamic properties of these species are described by the 7-coefficient NASA polynomials. For the
-containing species, data from the NASA database (McBride Citation2002), Goos et al. (Citation2005). and Rumminger et al. (Citation1999) are used. For the remaining species, the values from Konnov (Citation2019) are used.
We only consider the conversion of to
, which is the relevant oxide up to the maximum particle temperature (Thijs et al. Citation2022). We assume that mass and species transport is infinitely fast within the condensed phase
(Sun, Dobashi, Hirano Citation2000; Thijs et al. Citation2022) and only the energy equation has to be solved, which is given by
with ,
and
the density, specific heat at constant pressure and thermal conductivity of the condensed phase. The apparent heat capacity method (COMSOL Citation2019) is used to model the phase change of the iron particle. Temperature dependent densities are used for
(Hixson, Winkler, Hodgdon Citation1990; Watanabe et al. Citation1980) and FeO (Lee and Gaskell Citation1974; Saxena et al. Citation1993). A temperature dependent thermal conductivity is used and based on the values reported by Akiyama et al. (Citation1992) and Ho, Powell, and Liley (Citation1972). Thermodynamic properties are calculated with the NASA-7 polynomials with the coefficients obtained from the NASA database (McBride Citation2002).
Evaporation of the liquid iron droplet is taken into account via the saturated vapor pressure. The concentration of gaseous species on the particle surface can be written as
where and
is either
and
and
is the molar fraction in the condensed phase times the vapor pressure, stemming from Raoult’s law. The vapor pressure is a function of temperature and is based on thermochemical equilibrium calculations (Ning et al. Citation2021b). These calculations were performed with Cantera for pure
and
at
–
and
.
Lagrangian particle model
For the point particle model we extend the model of Hazenberg and van Oijen (Citation2021). The rate of change of and
are related to the rate of oxidation and the rate of evaporation via
with the mass of the particle,
the mass flux of oxygen to the particle,
the normalized Damköhler number (Hazenberg and van Oijen Citation2021) and
the stoichiometric mass ratio. Here,
.
The rate of change of the particle enthalpy is described by
with the convective heat flux,
the radiative heat flux,
the mass-specific enthalpy of the consumed oxygen evaluated at the particle temperature
and
the mass-specific enthalpy of the evaporated species.
The mass flux and heat flux can be described as
with ,
and
the density of oxygen, the mixture-averaged diffusion coefficient and the thermal conductivity derived at the film temperature and composition,
the gas temperature,
the mole fraction of oxygen in the gas phase and
and
the new Nusselt and Sherwood numbers. Here,
is calculated via the ideal gas law
with the molar mass of oxygen,
the pressure and
the universal gas constant.
In Section 3.1.2 we derive new Nusselt and Sherwood correlations which are accurate for low-Reynolds numbers and large temperature differences. We derive the transport properties at a reference temperature and composition
(Hubbard, Denny, Mills Citation1975)
In this work we will use the -film rule
as the reference temperature and composition. The Nusselt and Sherwood correlations are
with the Prandtl number and
the Schmidt number. A factor
is introduced to account for the effect on the transport properties due to the large temperature differences
with and
.
To model Stefan flow induced by the oxygen diffusion toward the particle, theory from droplet condensation/evaporation is used. The Nusselt and Sherwood numbers are corrected for Stefan flow via (Bird, Lightfoot, Stewart Citation2002; Both, Mira, Lehmkuhl Citation2022)
with the mass Spalding number defined as (Bird, Lightfoot, Stewart Citation2002)
Analogous to the mass Spalding number, a Spalding heat transfer number can be derived as (Bird, Lightfoot, Stewart Citation2002)
with and
the Lewis number.
The rate of evaporation can be described with and without evaporation induced Stefan flow. Since the mass flux of oxygen is much larger than the evaporate mass flux (Thijs et al. Citation2022), we can neglect the evaporation induced Stefan flow. Therefore, the evaporation rate becomes
with either
or
,
the evaporated mass transfer coefficient and
the molar fraction of the
or
vapor at the particle surface. The vapor molar fraction is determined according to EquationEquation 8
(8)
(8) .
The above described equations are solved within MATLAB, were we used the ode15s function for solving the differential equations.
Results
Boundary layer resolved model
The boundary layer resolved model is used to investigate the influence of several mentioned physical phenomena in order to derive modeling strategies for the Lagrangian point particle model. The Nusselt and Sherwood correlations are reviewed in flame-like conditions, with a focus on the reference temperature used to determine transport properties. Then, the 0D model is compared to the boundary-layer resolved model and the latter is used to investigate the effect of different modeling strategies.
In the analysis below, the effect of Stefan flow on the Nusselt and Sherwood correlations will be neglected. Both, Mira, and Lehmkuhl (Citation2022) investigated different methods subjected to flame-like conditions to incorporate Stefan flow effects in the heat mass transfer. They stated that the Bird’s correction, as described in Section 2, together with the Abramzon–Sirignano model, are the most relevant models to model the effect of Stefan flow.
Validation
First, simulations have been performed to investigate the effect of the domain size and the grid resolution on the obtained heat transfer coefficients. It is known that the effect of domain size becomes more important when the Reynolds number is decreased (Tajfirooz et al. Citation2021). we found that a domain of ,
,
with 120000 elements is sufficient to obtain an undisturbed flow when
, while we use
,
,
with around 720000 elements if
.
In order to verify the level of confidence of the used model, a validation of the program for different flow regimes is performed. Note that in Thijs et al. (Citation2022) we validated the physical model used for the combustion of iron particles. Here, we will use the boundary-layer resolved model to investigate the heat transfer coefficients, and compare it to existing well-established correlations. In the model, the particle is kept stationary while the gas flows across it. Therefore, the particle Reynolds number can be determined via
with the relative velocity,
the kinematic fluid viscosity determined at the reference temperature.
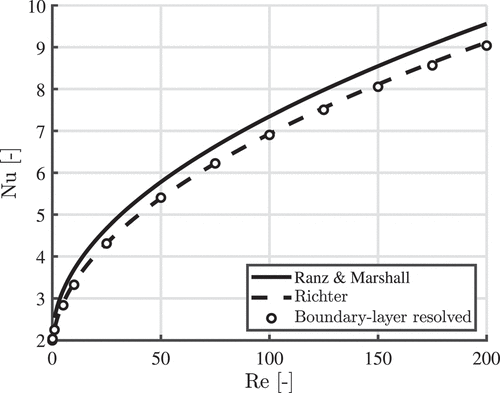
The numerically obtained Nusselt numbers are compared to the correlations proposed by Ranz and Marshall (Citation1952) (EquationEquation (1)(1)
(1) ) and Richter and Nikrityuk (Citation2012) (EquationEq 2)
(2)
(2) . Constant material properties corresponding to air at
are used and we consider a temperature difference of
between the particle and the gas. The results are shown in . Good agreement with the Richter correlation can be seen.
Nusselt and Sherwood correlations at large temperature differences
To enable modeling of single particle combustion without resolving the full boundary layers, reliable heat and mass transfer coefficients must be used. In the previous Section we showed that the boundary-layer resolved model shows good agreement with existing correlations in a large range of Reynolds number. However, particles of m or smaller are practically relevant for iron particle combustion, the particle Reynolds number will be in the lower Reynolds number regime, with
.
We perform steady simulations to investigate the heat transfer in the boundary layer for different Reynolds numbers. Since the mass transfer coefficient is analogous to the heat transfer coefficient, the analysis shown below also holds for the Sherwood number (Bird, Lightfoot, Stewart Citation2002). We consider different cases with ,
and
and
, which we will refer to as a cold flow. Also the opposite case is investigated, with
, to investigate the effect of a cold particle in a hot flow. The latter case is important before the thermal runaway happens for the iron particle, while the first case is of importance after thermal runaway.
The average heat transfer coefficient for the particle is calculated as
with the normal heat flux at the particle surface. Subsequently, the Nusselt number is calculated as
with the thermal conductivity determined at the reference temperature and composition.
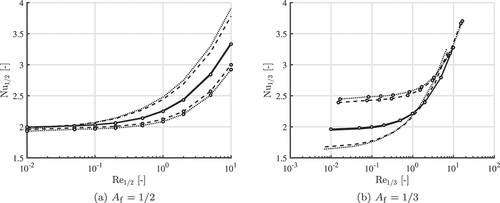
shows the Nusselt number correlations obtained with the boundary-layer resolved model in the case of a hot flow (curves with markers) and a cold flow (curves without markers) past an iron particle with diameter m. The Reynolds number and Nusselt number are determined at the same reference temperature, which is either the
-film or the
-film temperature rule. Three different temperature differences are used, namely
K (solid line),
K (dotted line) and
K (dashed line). For
it can be seen that the Nusselt correlations are sensitive to the large temperature differences in the higher Reynolds number regime, but are close to the theoretical limit of
in the lower Reynolds number regime. The opposite can be seen if
. This means that the
-film rule would be a wise choice if the particle Reynolds number is large. Then, the Nusselt and Sherwood numbers are independent of the temperature differences. However, if micron sized particles are considered, the Reynolds number is generally small. Therefore, we conclude that the
-film rule is the best rule to use when modeling the combustion of single iron particles, since it is independent of temperature and approaches
in the lower Reynolds number regime.
Figure 3. Nusselt correlation for different reference temperatures. Line represent either a hot gas flow (with markers) or a cold gas flow (without markers) past a iron particle with a particle diameter m. Temperature differences between the gas and the particle are: 10 K (solid line), 1200 K (dotted line) and 2200 K (dashed line).
Why the 1/2-file rule is more accurate in the low-Reynolds number regime can be demonstrated by investigating the 1D temperature profile in the boundary layer. When neglecting convection, the total heat conduction rate of a sphere can be written as (Liu et al. Citation2006)
with the thermal conductivity a function of temperature and written as
with constants and
. The average thermal conductivity in the range from
to
For air, the thermal conductivity scales almost linear with temperature (). If we substitute
, and rewrite the equation we find
which shows that the conduction of the film is equal to the conductivity at the -film temperature. Substituting EquationEquation (31)
(31)
(31) into EquationEquation (28)
(28)
(28) results in an expression of the heat flux, which is dependent on the average thermal conductivity.
From this we conclude that when neglecting convection, using the 1/2 rule will result in an accurate heat flux.
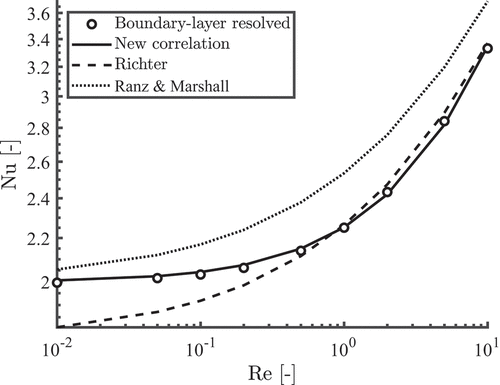
In it was shown that the correlation proposed by Richter and Nikrityuk (Citation2012) gives good agreement with the boundary layer resolved model for a large range of Reynolds numbers. However, this correlation does not approach the theoretical limit of if
. In order to come up with a Nusselt correlation which is accurate in the regime
and applicable to high temperature differences, a similar approach as Wittig, Nikrityuk, and Richter (Citation2017) and Richter and Nikrityuk (Citation2012) is used. Steady simulations are performed where a small temperature difference of 5 K is applied to a particle of 50 µm. By using small temperature difference the impact of the reference temperature is almost negligible. The following equation is then fitted through the obtained Nusselt number
with and
the fitting parameters, which are found equal to
and
, respectively. shows the results of the boundary-layer resolved model, the new proposed correlations and the correlations of Ranz and Marshall (Citation1952) and Richter and Nikrityuk (Citation2012). It can be seen that the new correlation is more accurate in this low Reynolds regime
than the existing correlations of Ranz and Marshall (Citation1952) and Richter and Nikrityuk (Citation2012).
To account for the changing thermophysical parameters in the boundary layer in the higher Reynolds number regime (), EquationEquation (33)
(33)
(33) is corrected with a factor
which is dependent on the temperature difference. A correction factor
, which is a function of the ratio
, can be fitted with EquationEquation (19)
(19)
(19) , with
and
.
Lagrangian point particle model
In this section, we present the results of a single iron particle burning in an -
and
-
atmosphere. The initial conditions are chosen such that the laser-ignited experiments as performed by Ning et al. (Citation2021a) are mimicked. First, the Lagrangian particle model is compared to the boundary layer resolved model. Then, the Lagrangian particle model is used to investigate the effect of temperature-dependent particle density, slip velocity and Stefan flow on the time to maximum temperature. If the size of the particle becomes too small, modeling the heat and mass transfer using the continuum approach becomes invalid (Liu et al. Citation2006). Therefore, we will focus on particles with a diameter of
µm to ensure that we are still in, or at least close to the continuum regime (Sundaram, Puri, Yang Citation2016).
Comparison between boundary layer resolved and Lagrangian particle model
Temperature profiles obtained with the boundary layer resolved model are compared to the Lagrangian particle models. We consider a 50 µm particle burning in an -
and
-
atmosphere, with
. The models include temperature-dependent particle density, slip velocity, Stefan flow and evaporation. shows this comparison of the temperature profiles between the boundary layer model and the particle model. The solid lines are obtained with the Nusselt and Sherwood correlations as proposed by EquationEquations (17
(17)
(17) , Equation18
(18)
(18) ). Due to the high initial particle temperature, the particle burns in the diffusion-limited regime and then reaches a maximum temperature of around
when
is fully oxidized into
. Two plateaus are visible in the temperature profile. The first plateau is due to the transition of
into
and the second plateau due to the transition of
into
. Good agreement between the boundary layer resolved model and the Lagrangian particle model is obtained, when using the
-film rule and the new proposed Nusselt and Sherwood correlations. Note that agreement is good for both the inert gases (argon or nitrogen), since the Nusselt and Sherwood correlations scales with the dimensionless Prandtl and Schmidt numbers.
Figure 5. Temperature profiles for a µm particle burning in (a) Nitrogen and (b) Argon with
. Temperature profiles are compared between the boundary layer resolved model (markers) and the Lagrangian particle model (line). The solid lines are obtained with the Nusselt and Sherwood correlations as proposed by EquationEquations (17)
(17)
(17) and (Equation18
(18)
(18) ), while the dashed line is obtained with the Ranz- Marshall correlations and the 1/3-rule.
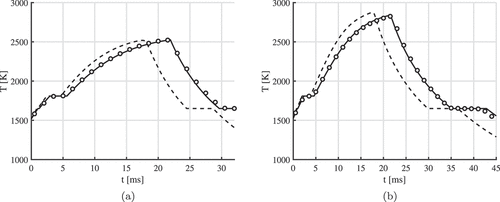
In Section 3.1.2 we showed that the -film rule is a wise choice. However, when modeling the combustion of micron-sized particles, the
-film rule is often used (Hazenberg and van Oijen Citation2021; Mi, Fujinawa, Bergthorson Citation2022; Ravi, de Goey, van Oijen Citation2022). The dashed lines in are obtained with the
-film rule and Ranz-Marshall correlations. It can be seen that one underestimates the time to maximum temperature when using these settings.
In the time to maximum temperature, the maximum temperature and the amount of iron evaporation as function of for a
µm particle obtained with the different models are stated. For the Lagrangian particle model, we distinguish again between the new proposed method (new correlation and
) and the previous used methods (Ranz-Marshall and
). We see that
decreases with an increasing oxygen concentration, while
increases. Since the particle is in a diffusion-limited regime up to the maximum temperature, an increasing oxygen concentration will enhance the oxygen flux toward the particle and therefore results in a lower
. At the same time, the particle temperature will increase with increasing oxygen concentration since the heat release rate is larger than the heat loss rate. Excellent agreement between the boundary-layer resolved model and the Lagrangian point particle model with the new proposed settings can be seen. When using the Ranz-Marshall correlation and
-film rule one consistently underestimates the time to maximum temperature, while it obtains good agreement with the maximum temperature. Therefore, it is of importance to use the
-film rule to model the mass and heat transport in a Lagrangian particle model.
Table 1. Comparison between the boundary-layer resolved model (BLR) and the Lagrangian particle model (0D) for a µm particle burning in
–
. We distinguish between the new proposed method (new correlation and
) and the previous used methods (Ranz-Marshall and
).
Effect of modeling strategies
Next, the effect of different modeling strategies on the time to maximum temperature of a µm iron particle in an
-
is investigated. We will systematically elaborate the model by including a temperature-dependent particle density, slip velocity and Stefan flow. In the 5 different cases are listed.
Table 2. Summary of test cases used to investigate the effect on .
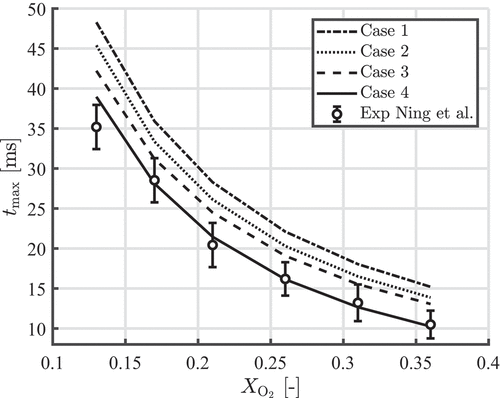
shows the time to maximum temperature as a function of the oxygen concentration for the five different cases. It can be seen that adding more physical phenomena improves the agreement with the experimentally obtained results of Ning et al. (Citation2021a, Citation2022). In Thijs et al. (Citation2022) we also compared the burn times of m particles, where we obtained excellent agreement. Here we will only focus on the results of
µm. We will use
as the reference composition to estimate the effect on
.
Using a temperature-dependent particle density instead of a constant particle density and neglecting slip and Stefan flow, will decrease by around
. The density of
and
decrease as a function of temperature, which means that the particle volume increases more than in the case of a constant density. A larger particle means a larger reacting surface area resulting in a smaller
.
Considering the effect of slip velocity on heat and mass transfer will decrease by another
. Slip will enhance the mass and heat transfer, resulting in larger
and
numbers. Since the terminal velocity of particles scales with the particle diameter, the effect of slip will be less when smaller particles are considered and larger when larger particles are considered.
Finally, in case 4 it is shown that modeling the oxygen induced Stefan flow will decrease by another
. Similar as slip, Stefan flow will enhance the mass transfer toward and heat transfer away from the particle. Note that the effect of Stefan flow increases when the oxygen concentration increases. For
,
decreases by
with respect to case 3, while it decreases by
with respect to case 3 for
.
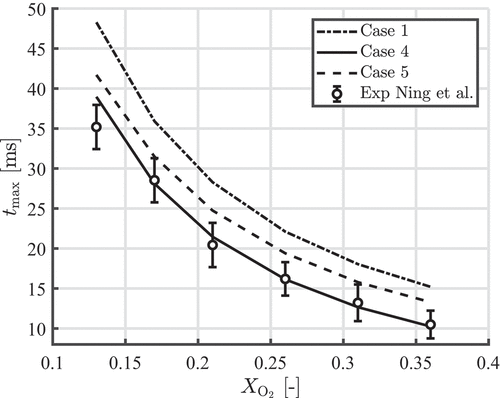
Ravi, de Goey, and van Oijen (Citation2022) showed that by using the -film rule, good agreement is obtained with the experiments of Ning et al. (Citation2021a, Citation2022). However, Ravi, de Goey, and van Oijen (Citation2022) neglected the effect of slip and Stefan flow (
), and did not take into account a temperature-dependent particle density. In we show the effect of the film rule on the temperature profile for a
µm particle burning
. Here, the results are shown for cases 1, 4 and 5. When comparing case 1 and case 5, it can be seen that using
will decrease the time to maximum temperature with around
. Furthermore, it can be seen that when neglecting physical phenomena like slip and Stefan flow, but using
, one will still obtain reasonable agreement with the experiments of Ning et al. (Citation2021a, Citation2022). However, different errors cancel each other. Note that the available experimental data is limited, and more independent experiments of single iron particle combustion in normal air are needed to further validate the model.
Conclusions and future directions
In this paper, a boundary layer resolved model is used to improve a 0D Lagrangian particle model for the first stage of diffusion-limited iron particle combustion. First, the boundary layer resolved model is used to derive new Nusselt and Sherwood correlations which apply to iron-particle combustion conditions. We added an extra term that accounts for the temperature difference between the particle and the gas, while we derive transport properties with the -film temperature.
The boundary layer resolved model is used to validate the Lagrangian point particle model. Good agreement between the boundary layer resolved model and the Lagrangian particle model is obtained, when using the new proposed Nusselt and Sherwood correlations. However, when using the Ranz Marshall correlation and the -film rule, one underestimates the time to maximum temperature of single metal particles.
With the point particle model, the effect of different modeling strategies on the time to maximum temperature of a µm laser-ignited iron particle is investigated. We systematically elaborated the model by including a temperature-dependent particle density, slip velocity and Stefan flow. Including all these effects decreases the time to maximum temperature by around
, and results in a good agreement with experiments. It is also shown that if one neglects physical phenomena like slip and Stefan flow, but uses the
-film rule instead of the
-film rule, still reasonable agreement is obtained with experiments, but different errors cancel each other.
In future research, the point particle model can be further improved. Other stages of combustion which are not diffusion limited could be investigated. The model could be elaborated with a two-film model to take into account the oxidation of the evaporated iron in the boundary layer. Furthermore, the Knudsen effect can be included to enable investigating the transition regime for smaller particles.
Acknowledgments
We would like to thank X. Mi for his valuable feedback. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under Grant Agreement no. 884916. and Opzuid (stimulus / European Regional Development Fund) Grant agreement No. PROJ-02594.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akiyama, T., H. Ohta, R. Takahashi, Y. Waseda, and J. Yagi. 1992. Measurement and modeling of thermal conductivity for dense iron oxide and porous iron ore agglomerates in stepwise reduction. ISIJ Int 32 (7):829–37. doi:10.2355/isijinternational.32.829.
- Bergthorson, J. M., S. Goroshin, M. J. Soo, P. Julien, J. Palecka, D. L. Frost, and D. J. Jarvis. 2015. Direct combustion of recyclable metal fuels for zero-carbon heat and power. Appl. Energy 160:368–82. doi:10.1016/j.apenergy.2015.09.037.
- Bird, R. B., E. N. Lightfoot, and W. E. Stewart. 2002. Transport phenomena. New York: J. Wiley.
- Both, A., D. Mira, and O. Lehmkuhl. 2022. Evaporation of volatile droplets subjected to flame-like conditions. Int. J. Heat Mass Transf 187:122521. doi:10.1016/j.ijheatmasstransfer.2022.122521.
- COMSOL. 2019. COMSOL multiphysics ® v. 5.5. Stockholm, Sweden: COMSOL AB. Reference Manual.
- Dreizin, E., A. Suslov, and M. Trunov. 1993. General trends in metal particles heterogeneous combustion. Combust Sci Technol 90 (1–4):79–99. doi:10.1080/00102209308907604.
- Ellendt, N., A. Lumanglas, S. I. Moqadam, and L. Mädler. 2018. A model for the drag and heat transfer of spheres in the laminar regime at high temperature differences. Int J Therm Sci 133:98–105. doi:10.1016/j.ijthermalsci.2018.07.009.
- Fiszdon, J. K. 1979. Melting of powder grains in a plasma flame. Int J Heat Mass Transf 22 (5):749–61. doi:10.1016/0017-9310(79)90122-4.
- Goos, E., A. Burcat, and B. Ruscic. 2005 Extended third millennium ideal gas andcondensed phase thermochemical database for combustion with updates from activethermochemical tables. German Aerospace Center. doi:10.2172/925269
- Hazenberg, T., and J. A. van Oijen. 2021. Structures and burning velocities of flames in iron aerosols. Proc Combust Inst 38 (3):4383–90. doi:10.1016/j.proci.2020.07.058.
- Hixson, R. S., M. A. Winkler, and M. L. Hodgdon. 1990. Sound speed and thermophysical properties of liquid iron and nickel. Phys. Rev. B 42 (10):6485–91. doi:10.1103/PhysRevB.42.6485.
- Ho, C. Y., R. W. Powell, and P. E. Liley. 1972. Thermal conductivity of the elements. J. Phys. Chem. Ref. Data 1 (2):279–421. doi:10.1063/1.3253100.
- Hubbard, G., V. Denny, and A. Mills. 1975. Droplet evaporation: Effects of transients and variable properties. Int J Heat Mass Transf 18 (9):1003–08. doi:10.1016/0017-9310(75)90217-3.
- Jayawickrama, T. R., N. E. L. Haugen, M. U. Babler, M. Chishty, and K. Umeki. 2021. The effect of Stefan flow on nusselt number and drag coefficient of spherical particles in non-isothermal gas flow. Int. J. Multiph. Flow 140:103650. doi:10.1016/j.ijmultiphaseflow.2021.103650.
- Konnov, A. A. 2019. Yet another kinetic mechanism for hydrogen combustion. Combust. Flame 203:14–22. doi:10.1016/j.combustflame.2019.01.032.
- Lee, Y. E., and D. R. Gaskell. 1974. The densities and structures of melts in the system Cao-“FeO”-SiO2. Metall. Mater. Trans. B 5 (4):853–60. doi:10.1007/BF02643138.
- Li, S., J. Huang, W. Weng, Y. Qian, X. Lu, M. Aldén, and Z. Li. 2022. Ignition and combustion behavior of single micron-sized iron particle in hot gas flow. Combust Flame 241:112099. doi:10.1016/j.combustflame.2022.112099.
- Liu, F., K. J. Daun, D. R. Snelling, and G. J. Smallwood. 2006. Heat conduction from a spherical nano-particle: Status of modeling heat conduction in laser-induced incandescence. Appl. Phys. B 83:355–82.
- McBride, B. J., 2002. NASA Glenn coefficients for calculating thermodynamic properties of individual species, NASA Technical Paper, National Aeronautics and Space Administration, John H. Glenn Research Center at Lewis Field.
- Mi, X., A. Fujinawa, and J. M. Bergthorson. 2022. A quantitative analysis of the ignition characteristics of fine iron particles. Combust Flame 240:112011. doi:10.1016/j.combustflame.2022.112011.
- Ning, D., Y. Shoshin, J. A. van Oijen, G. Finotello, and L. P. H. de Goey. 2021a. Burn time and combustion regime of laser-ignited single iron particle. Combust. Flame 230:111424. doi:10.1016/j.combustflame.2021.111424.
- Ning, D., Y. Shoshin, M. van Stiphout, J. A. van Oijen, G. Finotello, and L. P. H. de Goey. 2021b. Temperature and phase transitions of laser ignited single iron particle. Combust. Flame 236:111801. doi:10.1016/j.combustflame.2021.111801.
- Ning, D. 30 jun 2022 Experimental Investigation into Single Iron Particle Combustion (Eindhoven University of Technology)
- Poletaev, N., and M. Khlebnikova. 2020. Combustion of iron particles suspension in laminar premixed and diffusion flames. Combust Sci Technol 194 (7):1356–77. doi:10.1080/00102202.2020.1812588.
- Ranz, W., and J. Marshall. 1952. Evaporation from drops. Chem. Eng. Prog 48:173–80.
- Ravi, A., L. P. H. de Goey, and J. A. van Oijen. 2022. Flame structure and burning velocity of flames propagating in binary iron aerosols. Proc Combust Inst. Under Review.
- Richter, A., and P. A. Nikrityuk. 2012. Drag forces and heat transfer coefficients for spherical, cuboidal and ellipsoidal particles in cross flow at sub-critical Reynolds numbers. Int. J. Heat Mass Transf 55 (4):1343–54. doi:10.1016/j.ijheatmasstransfer.2011.09.005.
- Rumminger, M. D., D. Reinelt, V. I. Babushok, and G. T. Linteris. 1999. Numerical study of the inhibition of premixed and diffusion flames by iron pentacarbonyl. Combust. Flame 116 (1–2):207–19. doi:10.1016/S0010-2180(98)00033-9.
- Saxena, S. K., N. Chatterjee, Y. Fei, and G. Shen. 1993. Thermodynamic data on oxides and silicates. 1st ed. Berlin Heidelberg: Springer-Verlag.
- Schiemann, M., P. Fischer, and J. M. Bergthorson, 2017. Iron particles as carbon-neutral fuel in spray roasting reactors, in: Digital Proceedings of the 8th European Combustion Meeting, Dubrovnik, Croatia, April 2017, 487–92.
- Soo, M., X. Mi, S. Goroshin, A. J. Higgins, and J. M. Bergthorson. 2018. Combustion of particles, agglomerates, and suspensions – A basic thermophysical analysis. Combust. Flame 192:384–400. doi:10.1016/j.combustflame.2018.01.032.
- Sun, J. H., R. Dobashi, and T. Hirano. 2000. Combustion behavior of iron particles suspended in air. Combust Sci Technol 150 (1–6):99–114. doi:10.1080/00102200008952119.
- Sundaram, D. S., P. Puri, and V. Yang. 2016. A general theory of ignition and combustion of nano- and micron-sized aluminum particles. Combust. Flame 169:94–109. doi:10.1016/j.combustflame.2016.04.005.
- Tajfirooz, S., J. G. Meijer, J. G. M. Kuerten, M. Hausmann, J. Fröhlich, and J. C. H. Zeegers. 2021. Statistical-learning method for predicting hydrodynamic drag, lift, and pitching torque on spheroidal particles. Phys. Rev. E 103 (2):023304. doi:10.1103/PhysRevE.103.023304.
- Tang, F. D., S. Goroshin, A. Higgins, and J. Lee. 2009. Flame propagation and quenching in iron dust clouds. Proc Combust Inst 32 (2):1905–12. doi:10.1016/j.proci.2008.05.084.
- Thijs, L. C., C. E. A. G. van Gool, W. J. S. Ramaekers, J. A. van Oijen, and L. P. H. de Goey. 2022. Resolved simulations of single iron particle combustion and the release of nanoparticles. Proc Combust Inst. Under Review.
- Watanabe, S., Y. Tsu, K. Takano, and Y. Shiraishi. 1980. Density of pure iron in solid and liquid states. Japan Inst Metals 45 (3):242–49. doi:10.2320/jinstmet1952.45.3_242.
- Whitaker, S. 1972. Forced convection heat transfer correlations for flow in pipes, past flat plates, single cylinders, single spheres, and for flow in packed beds and tube bundles. AIChE J. 18 (2):361–71. doi:10.1002/aic.690180219.
- Wittig, K., P. Nikrityuk, and A. Richter. 2017. Drag coefficient and nusselt number for porous particles under laminar flow conditions. Int. J. Heat Mass Transf 112:1005–16. doi:10.1016/j.ijheatmasstransfer.2017.05.035.
- Wright, A., A. J. Higgins, and S. Goroshin. 2016. The discrete regime of flame propagation in metal particulate clouds. Combust Sci Technol 188 (11–12):2178–99. doi:10.1080/00102202.2016.1211877.