Abstract
A microstructured mixer, equipped with double-tube, swirl, and contraction flow channels, is proposed for the stable and continuous solvothermal synthesis of metal-organic framework (MOF) particles. The synthesis of Cu-BTC nanoparticles from copper nitrate and benzene 1,3,5-tricarboxylic acid ethanol solutions was conducted across temperatures ranging from 283 to 523 K. This study investigated the effects of temperature, flow rate, and mixing sequence on product yield and particle diameter. Comparative analysis of the results helped in highlighting the advantages of this mixer in preventing preheating of the starting solution and subsequent clogging.
1. Introduction
Metal-organic frameworks (MOFs) consist of porous structures composed of organic ligands and metal ions (Furukawa et al. Citation2014). By altering the combination, properties such as porosity, surface area, pore size, and adsorption can be finely tuned (Howarth et al. Citation2016). These materials find applications across various fields including gas separation/storage, catalysis, drug delivery, optics/electronics, and sensing (Meek et al. Citation2011; Yuan et al. Citation2018).
Scaling down MOFs to the nanometer level offers significant advantages in adsorption/desorption kinetics, catalytic activity, and bioavailability (Takahashi et al. Citation2015; Wang et al. Citation2018; Ehrling et al. Citation2021). Additionally, MOF nanoparticles (NPs) can serve as additives to enhance the separation properties and mechanical stability of polymer membranes for nanofiltration, owing to better controllability of pore structures and the stronger affinity of MOF organic ligands toward polymer chains (Sani et al. Citation2015; Wang et al. Citation2015). A stable and continuous production method for MOF NPs is highly desirable for such applications.
MOFs are typically synthesized via reactive crystallization by mixing metal salts with organic ligands under hydro- and solvothermal conditions (Yuan et al. Citation2018). To obtain smaller NPs, rapid mixing and heating are crucial for controlling competing processes of nucleation and growth. Flow synthesis methods offer rapid mixing and heating through the collision of multiple fluids in a micromixer, along with precise control of residence time in a microreactor (Mae et al. Citation2007; Wakashima et al. Citation2008).
Copper(II)-benzene-1,3,5-tricarboxylate, Cu3(BTC)2 (referred to as Cu-BTC), stands as a well-known and representative MOF, typically synthesized using copper(II) nitrate and benzene-1,3,5-tricarboxylic acid (BTC). Several flow methods for synthesizing Cu-BTC particles have been reported (Gimeno-Fabra et al. Citation2012; Faustini et al. Citation2013; Kim et al. Citation2013; Bayliss et al. Citation2014; Rubio-Martinez et al. Citation2014; Kubo et al. Citation2021). However, the obtained particle sizes often exceed 100 nm owing to challenges in achieving rapid mixing and heating to maintain crystallization under rate-limiting conditions. Further research is anticipated to produce smaller NPs below 100 nm through the optimization of micromixer and microreactor designs.
In this study, we conducted a continuous solvothermal synthesis of Cu-BTC NPs in ethanol using a novel microstructured mixer (Sue et al. Citation2015) designed specifically for rapid heating of a starting solution through mixing with two preheated fluids. The mixer incorporates swirl and contraction flow channels, alongside micrometer-scale channels, to enhance mixing rates by increasing the Reynolds number through a gradual decrease in inner diameter, coupled with the use of swirl flow (Wakashima et al. Citation2007). Additionally, pressure loss is reduced compared with conventional T-type micromixers. Furthermore, the mixer features a double-tube flow channel aimed at minimizing the temperature elevation of the starting solution and preventing associated precipitation prior to mixing with the preheated fluid. We investigated the effects of temperature, flow rate, and feed order of the starting solutions on particle diameter and its distribution, and subsequently discussed the advantages observed in the results.
2. Materials and Methods
2.1 Apparatus and procedure
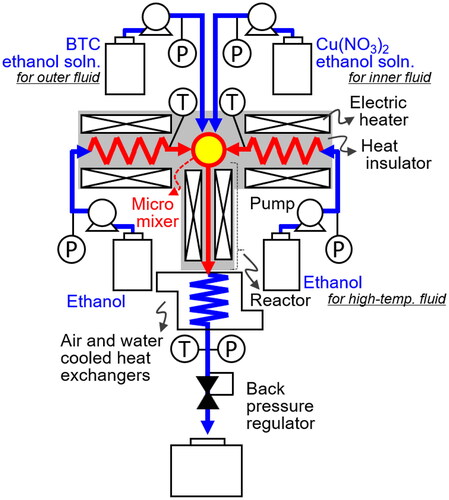
A schematic diagram illustrating the experimental setup for the continuous synthesis of Cu-BTC from Cu(NO3)2 and BTC ethanol solutions is depicted in . Detailed views of the microstructured mixer are presented in . The starting solutions were prepared by dissolving appropriate quantities of copper(II) nitrate trihydrate (Cu(NO3)2, purity 99.9%) and benzene-1,3,5-tricarboxylic acid (BTC, purity 99.0%) in ethanol (purity 100.0%) obtained from Wako Pure Chemical Industries, Ltd. The concentrations of Cu(NO3)2 and BTC in the reactor were maintained at 0.015 and 0.010 mol/L, respectively.
Figure 2. Detailed structure of the microstructured mixer: (a) overall structure; (b) vertical cross-section along the green line; (c) horizontal cross-section along the white line; (d) tip view of tube 2; (e) tip view of the rod; (f) upper end view of tube 3; and (g) lower end view of tube 3.
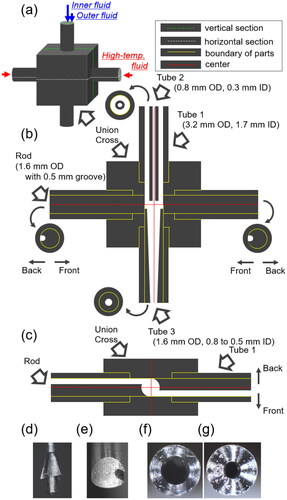
The Cu(NO3)2 and BTC ethanol solutions were introduced into the mixer center through the double-tube structure as inner and outer fluids, respectively (). To supply the outer fluid, a tube with an outer diameter (OD) of 3.2 mm and an inner diameter (ID) of 1.7 mm was used as tube 1 (). To supply the inner fluid, a tube with 0.8 mm OD and 0.3 mm ID was used as tube 2 (). To ensure centering the outlet of tube 2 in the mixer, four protrusions were incorporated around the tip of tube 2 (). This double-tube design aims to mitigate heating of the inner fluid through heat transfer via the mixer and tube 1 walls, as the outer fluid acts as a cooling solvent. Two streams of preheated ethanol were directed into the mixer at positions shifted away from the central axis, inducing swirl flow (). To realize the swirl flow, two rods having a 0.5 mm-deep groove was used in the mixer (). In addition, a tube with a narrowing ID from 0.8 mm to 0.5 mm was used to promote mixing using a contraction flow as tube 3 (). The rod and tube 3 were fixed inside the tube with the same dimensions as tube 1. These preheated ethanol streams served as the high-temperature fluid for the rapid mixing of the Cu(NO3)2 and BTC ethanol solutions and rapid solution heating to reaction temperatures, utilizing the swirl and contraction flow structures as depicted in and . Typical flow rates for each high-temperature fluid, inner fluid, and outer fluid were set at 40, 10, and 10 mL/min, respectively. Two total flow rate conditions, 100 and 25 mL/min, maintaining the same flow rate ratio, were examined to assess mixer performance. In all experiments, pure ethanol was initially fed from each pump, and after achieving a steady state at the designated temperature and pressure, the two feed fluids were changed to Cu(NO3)2 with BTC solutions.
The fluid temperature after mixing all the fluids was adjusted by controlling the temperature of the preheated ethanol, while the reactor was heated to the desired reaction temperature. In this study, the reaction temperature ranged from 283 to 523 K. The apparatus was maintained at 10 MPa using a back pressure regulator. Mean residence times were estimated based on the total flow rate, reactor volume, and ethanol density at the given temperature and pressure (Schroeder et al. Citation2014). The experimental conditions are summarized in .
Table 1. Experimental conditions for continuous Cu-BTC synthesis.
Effluents passing through the back pressure regulator underwent immediate filtration using hydrophilic PTFE filters (0.45 μm) for pressure filtration. The filtrates were diluted with a 0.1 mol/L HNO3 aqueous solution for quantitative analysis of remaining Cu ions. Residues on the filter were washed with ethanol and subsequently dried at 336 K for 24 h in air for X-ray diffraction (XRD) analysis. Effluents were also deposited onto TEM microgrids and allowed to dry at around 293 K for 24 h under vacuum for scanning electron microscopy (SEM) observation.
2.2 Analyses
The crystal structures of the residue were analyzed via XRD using a MiniFlex II instrument from Rigaku, with CuKα radiation. Crystallite diameter was calculated employing the Scherrer equation, considering the full width at half-maximum of the main peak, peak position, and wavelength. Observations of the residue were conducted using SEM with a S-4800 instrument from Hitachi. The average particle diameter (APD) and standard deviation (SD) were determined by measuring the sizes of 200 particles across multiple SEM images. The concentration of the Cu ionic species remaining in the effluents was quantified using microwave plasma atomic emission spectroscopy (MP-AES) with a 4200 instrument from Agilent to ascertain the yield of solid products.
3. Results and Discussion
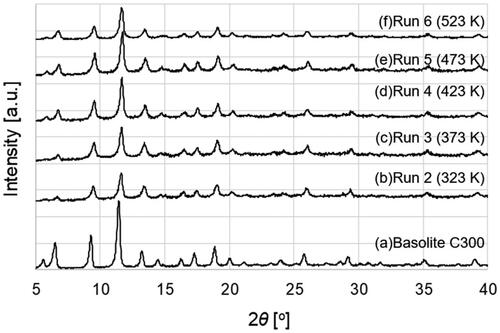
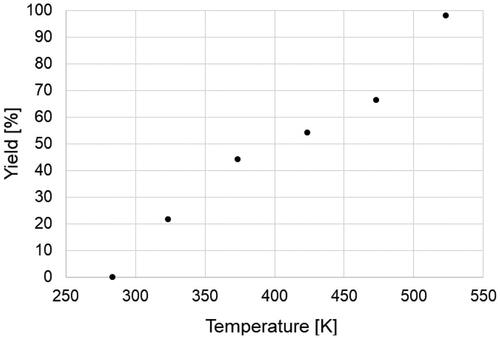
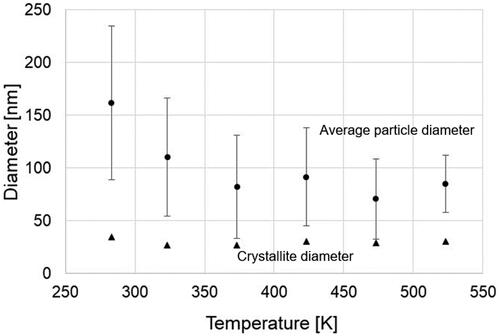
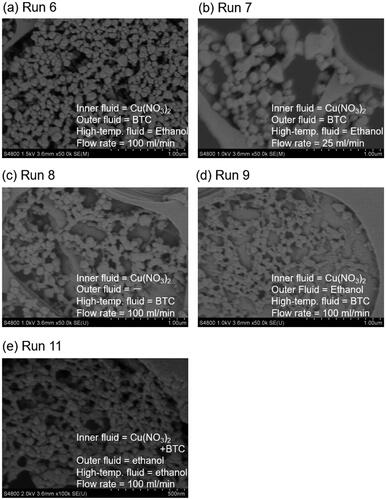
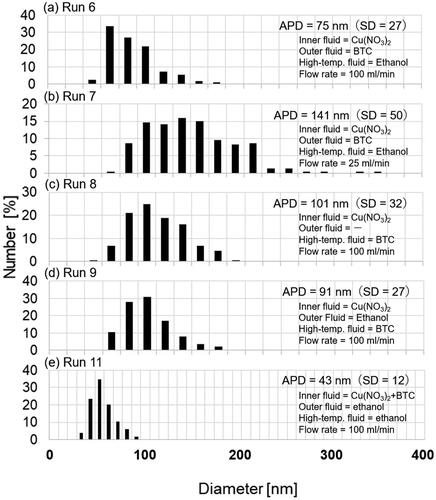
The typical XRD patterns of the products and Cu-BTC powder (Basolite C300) purchased from Sigma-Aldrich are illustrated in . All peaks of the products were identified as Cu-BTC. The yields of the products increased with rising temperature from 283 K (run 1) to 523 K (run 6), reaching approximately 98% at 523 K, as depicted in , despite the decrease in residence time from 0.86 s to 0.49 s. Crystallite diameters of the product ranged from 26 to 34 nm, with no significant trend observed with temperature variation, as shown in . However, the APD gradually decreased from 162 to 75 nm with increasing temperature.
At lower temperatures, the low yield and significant difference between crystallite and APD indicate not only the gradual formation of a few NPs through nucleation but also the concurrent aggregation growth after mixing due to the low reaction rate and low degree of supersaturation. In contrast, at higher temperatures, numerous NPs rapidly form and crystallize due to the high reaction rate and high degree of supersaturation. Consequently, subsequent aggregation growth can be avoided, leading to the production of smaller particles.
In this system, a synthesis experiment at a low total flow rate of 25 mL/min and 523 K was also conducted. and show the SEM images of the products and particle size distribution, respectively. The decrease in flow rate from 100 mL/min (run 6) to 25 mL/min (run 7) increased the APD from 75 to 141 nm, as shown in , while maintaining the crystallite diameter below 30 nm and the yield around 98%. The increase in particle diameter can be attributed to the decrease in mixing and heating efficiency.
In the following section, we delve into the effects of the supply procedure of Cu(NO3)2 solution, BTC solution, and ethanol on particle diameter. Four additional procedures were examined during synthesis at 523 K, while concentrations of Cu(NO3)2 and BTC in the reactor and total flow rate remained constant. These conditions are outlined in (runs 8–11). In all runs, the flow rate ratio of the inner and outer fluids to each high-temperature fluid is kept at 0.5.
First, BTC ethanol solution and pure ethanol were supplied from the high-temperature fluid line and outer fluid line, respectively (run 9). For comparison, synthesis without pure ethanol feed as the outer fluid was conducted (run 8). For this case, nothing flows into the outer tube. In these syntheses (runs 6, 8, 9), no significant differences in crystallite diameter and yield were observed. By shifting the feed of the BTC solution from the outer fluid line to the high-temperature fluid line, the APD slightly increased from 75 to 91 nm. This suggests that supplying the BTC into the outer fluid line is effective in producing smaller NPs, possibly due to the more rapid mixing of Cu(NO3)2 with BTC and subsequent nucleation. Additionally, APD without pure ethanol feed into the outer fluid line increased to 101 nm, indicating the importance of pure ethanol feed into the outer fluid line for producing smaller NPs. Notably, no remarkable pressure increase or clogging was observed in the experiments discussed above.
Second, to enhance the mixing rate of Cu(NO3)2 with BTC, Cu(NO3)2 and BTC ethanol solution were fed into the T-type micromixer (0.33 mm ID, not shown in ), and subsequently, the mixed solution was fed into the inner fluid line. The residence time in the tube from the T-type mixer to the inner fluid line was estimated to be 0.03 s based on the flow rate and the volume in the tube. Prior to this attempt, mixing Cu(NO3)2 with BTC solution was examined in a glass bottle and some products were produced. Therefore, a feeding of pre-mixed solution was not performed. After an additional pump was introduced into the apparatus, pure ethanol was supplied into the outer fluid and high-temperature fluid lines (run 11). For comparison, synthesis without pure ethanol feed as the outer fluid was also conducted (run 10). After changing the feed fluids to Cu(NO3)2 and BTC solutions, the pressure sharply increased for run 10 probably due to heterogeneous nucleation on the inner surface of heated tube 2 and additional growth of the nucleated particles before mixing with the high-temperature fluid, and the effluent with the products could not be recovered. In contrast, continuous synthesis could be performed for run 11, yielding small NPs with an APD of 43 nm. These results underscore the significant advantage of the developed microstructured mixer in preventing preheating of the starting solution and subsequent clogging.
4. Conclusion
A microstructured mixer, featuring double-tube, swirl, and contraction flow channels, was designed for continuous synthesis of nanoparticles under high-temperature and high-pressure conditions. Solvothermal synthesis of Cu-BTC nanoparticles in ethanol was conducted to investigate the effects of temperature, flow rate, and feed procedure on the properties of the obtained nanoparticles. The results underscored several advantages of the mixer, including the acceleration of homogeneous nucleation, prevention of clogging in the mixer channels, and continuous production of small Cu-BTC nanoparticles with an average particle size of 43 nm. Feature comparison of the obtained smaller nanoparticles with commercial Cu-BTC will be explored in a future study. To apply the proposed mixer to new production processes of various functional particles, further studies using a computational fluid dynamics simulation are desired to clarify the effect of double-tube, swirl, and contraction flows and to optimize the mixer structure.
References
- Bayliss PA, Ibarra IA, Pérez E, Yang SH, Tang CC, Poliakoff M, Schröder M. 2014. Synthesis of metal-organic frameworks by continuous flow. Green Chem. 16:3796–3802. doi: 10.1039/C4GC00313F.
- Ehrling S, Miura H, Senkovska I, Kaskel S. 2021. From macro- to nanoscale: finite size effects on metal-organic framework switchability. Trends in Chem. 3:291–304.
- Faustini M, Kim J, Jeong GY, Kim JY, Moon HR, Ahn WS, Kim DP. 2013. Microfluidic approach toward continuous and ultrafast synthesis of metal-organic framework crystals and hetero structures in confined microdroplets. J Am Chem Soc. 135:14619–14626. doi: 10.1021/ja4039642.
- Furukawa S, Reboul J, Diring S, Sumida K, Kitagawa S. 2014. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale. Chem Soc Rev. 43:5700–5734. doi: 10.1039/c4cs00106k.
- Gimeno-Fabra M, Munn AS, Stevens LA, Drage TC, Grant DM, Kashtiban RJ, Sloan J, Lester E, Walton RI. 2012. Instant MOFs: continuous synthesis of metal-organic frameworks by rapid solvent mixing. Chem Comm. 48:10642–10644.
- Howarth AJ, Liu YY, Li P, Li ZY, Wang TC, Hupp J, Farha OK. 2016. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat Rev Mat. 1:15018.
- Kim KJ, Li YJ, Kreider PB, Chang CH, Wannenmacher N, Thallapally PK, Ahn HG. 2013. High-rate synthesis of Cu-BTC metal-organic frameworks. Chem Comm. 49:11518–11520.
- Kubo M, Ishimura M, Shimada M. 2021. Improvement of production efficiency of spray-synthesized HKUST-1. Adv Powder Technol. 32:2370–2378. doi: 10.1016/j.apt.2021.05.024.
- Mae K, Suzuki A, Maki T, Hakuta Y, Sato H, Arai K. 2007. A new micromixer with needle adjustment for instant mixing and heating under high pressure and high temperature. J. Chem. Eng. Jpn. 40:1101–1107.
- Meek ST, Greathouse JA, Allendorf MD. 2011. Metal-organic frameworks: a rapidly growing class of versatile nanoporous materials. Adv Mater. 23:249–267. doi: 10.1002/adma.201002854.
- Rubio-Martinez M, Batten MP, Polyzos A, Carey KC, Mardel JI, Lim KS, Hill MR. 2014. Versatile, high quality and scalable continuous flow production of metal-organic frameworks. Sci Rep. 4:5443. doi: 10.1038/srep05443.
- Sani NAA, Lau WJ, Ismail AF. 2015. Polyphenylsulfone-based solvent resistant nanofiltration (srnf) membrane incorporated with copper-1,3,5-benzenetricarboxylate (Cu-BTC) nanoparticles for methanol separation. RSC Adv. 5:13000–13010. doi: 10.1039/C4RA14284E.
- Schroeder JA, Penoncello SG, Schroeder JS. 2014. A fundamental equation of state for ethanol. J Phys Chem Ref Data. 43:043102.
- Sue K, Hakuta U, Furuya T, inventors; National Institute of Advanced Industrial Science and Technology, assignee. 2015. Mixing device of high temperature and high pressure fluids. Japan patent JP5649066B2.
- Takahashi A, Minami N, Tanaka H, Sue K, Minami K, Parajuli D, Lee K-M, Ohkoshi S-I, Kurihara M, Kawamoto T. 2015. Efficient synthesis of size-controlled open-framework nanoparticles fabricated with a micro-mixer: route to the improvement of Cs adsorption performance. Green Chem. 17:4228–4233. doi: 10.1039/C5GC00757G.
- Wang SZ, McGuirk CM, d‘Aquino A, Mason JA, Mirkin CA. 2018. Metal-organic framework nanoparticles. Adv Mater. 30:e1800202. doi: 10.1002/adma.201800202.
- Wang L, Fang M, Liu J, He J, Deng L, Li J, Lei J. 2015. The influence of dispersed phases on polyamide/ZIF-8 nanofiltration membranes for dye removal from water. RSC Adv. 5:50942–50954. doi: 10.1039/C5RA06185G.
- Wakashima Y, Suzuki A, Kawasaki S, Matsui K, Hakuta Y. 2007. Development of a new swirling micro mixer for continuous hydrothermal synthesis of nano-size particles. J Chem Eng Jpn. 40:622–629.
- Wakashima Y, Hatakeda K, Kawasaki S, Suzuki A. 2008. Performance evaluation of a high pressure microtube as a high-speed heating device for supercritical state generation. J Chem Eng Jpn. 41:76–83.
- Yuan S, Feng L, Wang KC, Pang JD, Bosch M, Lollar C, Sun YJ, Qin JS, Yang XY, Zhang P, et al. 2018. Stable metal-organic frameworks: design, synthesis, and applications. Adv. Mater. 30:1704303.