Abstract
The present study investigated whether the duration of the first tapping bout, which could also be considered ‘the priming’, would play a role for the occurrence of the behavioral phenomenon termed repeated bout rate enhancement. Eighty-eight healthy individuals were recruited. Sixty-three of these demonstrated repeated bout rate enhancement and they were assigned to two different groups, which performed either active or passive tapping as priming. The durations of the first tapping bouts, which acted as priming, were 20, 60, 120, and 180 s. Following the first bout there was a 10 min rest and a subsequent 180 s tapping bout performed at freely chosen tapping rate. Vertical displacement and tapping force data were recorded. Rate enhancement was elicited independently of the duration of the first bout in both groups. Rate enhancement occurred without concurrent changes of the magnitude of vertical displacement, time to peak force, and duration of finger contact phase. The peak force was reduced when 180 s of tapping had been performed as priming. The increased tapping rate following priming by as little as 20 s active or passive tapping, as observed here, is suggested to be a result of increased net excitability of the nervous system.
Introduction
The capacity to perform voluntary stereotyped rhythmic movements is of great importance for humans to function well. Examples of such movements include walking, running, pedaling, and finger tapping. Improved understanding of the control and behavior of such rhythmic movements can in a long-term perspective contribute to the improvement of motor treatment, rehabilitation, function, and performance of injured and healthy humans. Our current understanding of human voluntary stereotyped rhythmic movements is that such movements are considered to be controlled by spinal neural networks, termed central pattern generators (CPGs), assisted by tonic supraspinal input, and afferent feedback (Grillner, Citation2009; MacKay-Lyons, Citation2002; Prochazka & Ellaway, Citation2012; Zehr et al., Citation2004; Zehr & Duysens, Citation2004). Briefly, it has been argued that CPGs act as a component for the generation and modulation of rhythmic movements in humans (Burke et al., Citation2001, Zehr et al., Citation2004, Zehr, Citation2005). Besides, it has been described that sensory signals play an important role for the nervous system’s generation and modulation of rhythmic movement (Grillner, Citation2009, Frigon, Citation2017).
The precise inter-relationship between CPG-activity, central descending drive, and afferent feedback in the human nervous system is unresolved (Dimitrijevic et al., Citation1998; Zehr, Citation2005). Studies applying, e.g. voluntary pedaling (Hansen & Ohnstad, Citation2008, Stang et al., Citation2016; Sakamoto et al., Citation2007) and finger tapping (Hansen et al., Citation2015; Mora-Jensen et al., Citation2017; Shima et al., Citation2011) are performed to increase our understanding. Studies of the mechanisms underlying CPG-generated voluntary rhythmic movement in healthy humans are obviously challenged by limited access to the spinal cord (Dietz, Citation2003; Zehr, Citation2005). However, in order to further increase our knowledge of human rhythmic movement, analysis of motor behavior can be applied (Goulding, Citation2009; Klarner & Zehr, Citation2018; Schlinger, Citation2015).
It is a rather well known phenomenon that in a synchronization-continuation finger tapping paradigm (i.e. when participants are paced by a metronome at a given rate for a short period of e.g. 10 s and then try to continue the rate for a prolonged period without the pace), the tapping rate drifts either to higher or lower rates (Collyer et al., Citation1992; Madison, Citation2001) depending on the initial tapping rate. We have previously found a similar phenomenon during prolonged index finger tapping at freely chosen tapping rate in a large (n = 102) group of participants (Emanuelsen et al., Citation2019). A different rate phenomenon, which has been observed has been termed repeated bout rate enhancement (RBRE). RBRE can only be revealed during repeated bouts of finger tapping at freely chosen rates, as reported by Hansen et al. (Citation2015).
Briefly, the phenomenon comprises of an increased freely chosen finger tapping rate in the second of two consecutive tapping bouts of each 180 s, separated by a 10 min rest period. Thus, the first tapping bout can be considered to act as an initial kind of ‘priming’ of a second tapping bout. The RBRE phenomenon was recently replicated (Emanuelsen et al., Citation2018; Mora-Jensen et al., Citation2017). It has been speculated, that an increase of the freely chosen finger tapping rate in the second bout reflects an increase of CPG-mediated movement rate output (Hansen & Ohnstad, Citation2008; Shima et al., Citation2011). It follows that an increased CPG-mediated movement rate might be caused by an increased net excitability of supraspinal centers, an increased net excitability of spinal networks, or a combination of the two conditions (Hansen et al., Citation2015; Mora-Jensen et al., Citation2017). For clarity, the formulation of increased net excitability includes both the possibility of an increase in facilitation of supraspinal structures, CPGs, and motoneurons as well as a decrease in inhibitory input to the mentioned parts of the nervous system. Furthermore, a recent study investigated the effect of passive finger tapping (obtained by using a machine to move the finger) on RBRE (Emanuelsen et al., Citation2018). Based on that study, it was suggested that sensory feedback alone during the first bout was sufficient to increase the net excitability and result in an increased tapping rate in a subsequent bout of freely chosen tapping. However, several aspects on RBRE remain unresolved. For example, is elicitation of RBRE subjected to a dose (duration of priming)-response (RBRE) relationship? Further, is a potential dose-response relationship similar whether passive or active tapping is performed in the first bout?
The phenomenon of RBRE appears to be similar to what has been termed ‘repetition priming’ (Cropper et al., Citation2014; Cropper et al., Citation2017; Siniscalchi et al., Citation2016). Cropper et al. (Citation2014) characterized repetition priming as increased performance when behavior is repeated. It has been reported that episodic induction of the feeding motor program in Aplysia results in dynamic reconfiguration of CPG network activity, through intrinsic neuromodulators that exert effects, which summate and persist (Cropper et al., Citation2017). Further, it has been proposed that neuromodulators can exert effects at different timescales, from short-term adjustments of neuronal excitability and synaptic function to persistent long-term regulation (Nadim & Bucher, Citation2014). For example, it has been shown that 30 s of electrical stimulation of cerebral-buccal interneurons, which activate and/or modulate the feeding CPG, which drives rhythmic motor output of the feeding motor program in Aplysia, results in an increased cycle rate of the rhythmic ingestion buccal motor program, although not persistent for more than 2 min (Sánchez & Kirk, Citation2000, Citation2002). The findings by Sánchez and Kirk (Citation2000, Citation2002) may suggest that a priming duration of more than 30 s could be required for inducing increased net excitability in the nervous system, persisting for up to 10 min, as observed in RBRE. To elucidate that, we performed a pilot study prior to the present study. The data from the pilot study showed absence of RBRE for a group of 18 individuals who performed 30 s of priming, in form of freely chosen tapping, in an initial tapping bout, which was followed by 10 min rest and a second bout consisting of 180 s freely chosen tapping. Consequently, we considered it likely that a certain minimal duration of priming (above 30 s) would be required to provide increased net excitability of the nervous system, which is thought to be responsible for elicitation of RBRE.
The aim of the present study was to test the hypothesis that there is a dose-response relationship between the duration of priming and the magnitude of RBRE. The dose of priming ranged from 20 to 180 s of active or passive tapping. As a part of the hypothesis, we expected that more than 20 s of priming would be needed to elicit RBRE.
Methods
Participants
A total of 88 healthy individuals (37 men, 51 women, height: 1.74 ± 0.09 m, body mass: 72.7 ± 12.3 kg, age: 25.6 ± 5.3 years) were recruited for the present study. Handedness (78 right-handed, 10 left-handed) was self-reported. The participants received written and oral information about the procedures of the study as well as the overall aim. Still, the participants were not informed about the specific aims and hypotheses of the study, with the intention to avoid any particular conscious control of the performed finger tapping. Exclusion criteria were: 1) any history of neural or musculoskeletal diseases or disorders, and 2) recent exposure to considerable execution of rhythmic movements with their fingers, such as playing computer games or playing an instrument, more than one hour weekly. The participants were informed not to consume alcohol or euphoric substances during the final 24 hr before testing. Additionally, they were informed not to consume coffee during the final 3 hr before testing. Written informed consent was obtained from the participants. The study conformed to the standards set by the Declaration of Helsinki and the procedures were approved by The North Denmark Region Committee on Health Research Ethics (N-20170017).
Overall Design
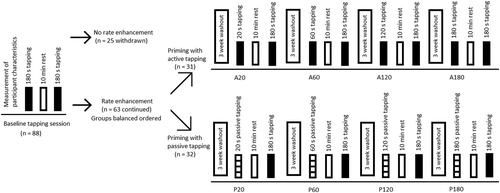
For the present study, a repeated measures design was applied. Each participant reported to the laboratory five times in total. The five attendances were separated by three to four week washout periods (Hansen et al., Citation2015; Hansen & Ohnstad, Citation2008; Sardroodian et al., Citation2016). During each of the five attendances, a single test session was performed. illustrates the experimental design. At the first attendance, the participant performed a baseline tapping bout to determine the freely chosen tapping rate. Subsequently, the participant had a 10-min rest period followed by a second tapping bout. Participants who showed RBRE from the first to the second bout in the baseline tapping session continued to participate in the study. Following the baseline tapping session, the participants were assigned, in a balanced fashion, to a group performing either active priming or a group performing passive priming. The balancing was performed to create similar group averages in relation to the relative tapping rate enhancement. Both groups had to perform four additional tapping sessions. For detailed description of the applied test protocols during the different test sessions, the reader is referred to the text below. The following four test sessions were performed in a counterbalanced order, within each group. It applies to all five test sessions that the participant reported to the laboratory at the same time of the day, to avoid any potential influence of circadian rhythm on finger tapping rate (Moussay et al., Citation2002). Additionally, there was no warm-up or familiarization before testing, with the purpose to prevent any form of priming before performing the first tapping bout.
Figure 1. Illustration of the experimental design. The sessions including active tapping as priming (A20, A60, A120, and A180) were counterbalanced. Besides, the sessions including passive tapping as priming (P20, P60, P120, and P180) were counterbalanced. In total, data from 28 and 29 participants in the active and passive group, respectively, were analyzed (for further details see the results section).
Baseline Tapping Session
The baseline tapping session was started by determining the participant’s age, body height, and body mass. Next, a demonstration was done by the experimenter on how to perform finger tapping and on the test procedure in general. The participant was shown and explained how to perform tapping with the index finger of the right hand at a freely chosen rate, while the remaining four fingers of the right hand were relaxed, extended and in contact with the table (Emanuelsen et al., Citation2018; Hansen et al., Citation2015; Mora-Jensen et al., Citation2017; Sardroodian et al., Citation2016). It was emphasized to the participant that tapping was not required to be performed at a constant rate or as fast as possible, but rather at “a comfortable rhythm” or at “an individually preferred rhythm” while at the same time “thinking about something else”. After the demonstration, a light emitting-diode (LED) tracker, a part of a motion capture system (Standard VZ-4000v, Phoenix Technologies Inc., Burnaby, BC, Canada), was attached to the midpoint of the nail of the participant’s index finger of the right hand. Next, the participant was comfortably seated in an office chair in the test position. During all tapping bouts, the participant assumed a standardized test position. The participant was instructed to keep the palm flat on the table. The participant’s back was straight, while shoulder and elbow joints were flexed approximately 50° and 45°, respectively. The lower arm was resting on the table. For detailed setup, including an illustration of the test position, the reader is referred to Sardroodian et al. (Citation2016).
Following the demonstration and preparation, a 180-s tapping bout was performed at a freely chosen tapping rate. Finger tapping was performed on a force transducer (FS6–250, AMTI, Watertown, MA, USA). Subsequent to the first tapping bout, the participant had a 10-min rest period. The rest period was followed by a second 180-s tapping bout performed at a freely chosen tapping rate. At the end of the baseline tapping session, the participant was familiarized with a custom built machine used to move the finger and perform passive tapping (Emanuelsen et al., Citation2018).
Participants who showed RBRE from the first to the second bout in the baseline tapping session were selected for participation in four additional tapping sessions applying either active or passive tapping as priming. In line with previous work by Emanuelsen et al. (Citation2018), a criterion of a minimum increase of 3% of the freely chosen tapping rate from the first to the second bout was applied.
Active Tapping as Priming
For this, there were four different test sessions performed, on four separate days, in a counterbalanced order. Each session consisted of two bouts of active, freely chosen, tapping. The duration of the first bout was either 20 (A20), 60 (A60), 120 (A120), or 180 s (A180). A 10-min rest period followed the first bout. The session was finalized by a 180-s bout of freely chosen tapping (equivalent to the A180 bout). See also . It applies to all four test sessions that they were initiated by a demonstration of the test procedure done by the experimenter. Thereafter, the LED-tracker was mounted as described for the baseline tapping session. Then, finger tapping was performed on the force transducer.
Passive Tapping as Priming
For this, there were also four different test sessions performed, on four separate days in a counterbalanced order. Each session consisted of a first bout of passive tapping. For this, passive tapping constituted of an imposed approximately tapping-like movement of the index finger while the participant was instructed to abstain from an activation of the involved muscles to avoid interference with the imposed movement. The duration of the first bout was either 20 (P20), 60 (P60), 120 (P120), or 180 s (P180). A 10-min rest period followed the first bout. The session was finalized by a 180-s bout of freely chosen tapping on the force transducer. See also . It applies to all four test sessions that they were initiated by a demonstration of the test procedure done by the experimenter. Thereafter, the LED-tracker was mounted as described for the baseline tapping session. Then, passive finger tapping in the first bout was applied to the index finger using a custom-built machine with a rocker arm (see Emanuelsen et al. (Citation2018) for photo and detailed information). The participant was instructed to “relax as much as possible” while the tip of the right index finger was placed at the end of the rocker arm, in conformity with Emanuelsen et al. (Citation2018). The vertical displacement during the passive tapping was set to 24 mm, based on previous findings of vertical displacement during tapping (Mora-Jensen et al., Citation2017). The tapping rate for each participant during the passive tapping bout corresponded to the average tapping rate that the participant had applied during the first tapping bout in the baseline tapping session.
Data Recordings and Analyses
Tapping Rate and Force
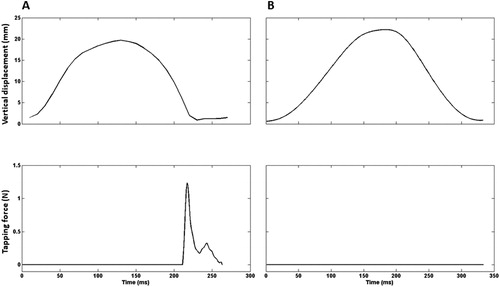
The force transducer was checked for accuracy and linearity before the beginning of each test session, using a range of fixed loads. Tapping force was measured in the vertical direction. The force signal was amplified 4000 times, analogue low-pass filtered at 1050 Hz, and digitalized using a 12 bits NI BNC-2090A A/D-board (National Instruments, Austin, TX, USA). The entire duration of the tapping bouts was sampled at 2000 Hz. The force recordings were digitally low-pass filtered at 200 Hz. The recordings were stored and visualized using a Lab-VIEW-based (National Instruments Co., Austin, TX, USA) custom-programmed software (Mr. Kick III software, Knud Larsen, Aalborg University, Aalborg, Denmark). Force recordings were analyzed using a custom-written MATLAB script developed for a previous study (Emanuelsen et al., Citation2018). Parameters were extracted on a tap-to-tap basis and the averages were calculated across the entire duration of tapping bouts. Briefly, a baseline force value was determined as the mean transducer output 1 s just before the tapping started. After the recording, the baseline output was subtracted from the signal obtained for each tap during the tapping bout, and the initiation of each tap was determined as the last time the signal crossed the baseline force value before the force increased due to the finger contact. The end of the period of finger contact was determined as the first time following the finger contact the force decreased below the baseline force value again. For a representative recording example of an individual tap see . The following variables were calculated for each tapping bout: (a) Tapping rate (taps min−1) was calculated as 60 s divided by the time (in s) between two consecutive force onsets. (b) Peak force (N) was determined as the difference between the maximal force value detected during the contact time and the reference force value. (c) Time to peak force (ms) was determined as the time from the force onset to the peak force during each tap. (d) Duration of finger contact phase (ms) was determined as the time between force onset and force offset. Force onset was determined as the time point of initial finger contact. Force offset was determined as the time point where the force returned to the baseline force value.
Figure 2. Representative recordings of individual taps from a single participant. Upper row: index finger displacement in the vertical direction (mm). Lower row: tapping force (N). Left column (A): active tapping. Right column (B): passive tapping. Each cycle was defined to begin when the tip of the finger was at its lowest point during each tap.
Vertical Displacement
The motion capture system was calibrated to define a 3D scaled local coordinate system. Vertical displacement of the fingertip in the sagittal plane was measured from the vertical coordinate of the LED-tracker. Data were sampled at 100 Hz, synchronously with the tapping force, using VZSoftTM software (Phoenix Technologies Inc., Burnaby, BC, Canada). Data were analyzed using MATLAB version R2013a (The MathWorks, Inc., Natick, MA, USA). For this analysis, the custom-written script was used to detect local maxima and minima on the position trace across the entire duration of the tapping bouts. Vertical displacement (mm) for the active situation was calculated by subtracting the minimum value from the maximum value for each tap, determined by the force trace. For the passive tapping condition, vertical displacement was calculated by subtracting the minimum value from the maximum value for each tap, determined by the position trace. Averaged values of vertical displacement across the bouts were computed prior to statistical analyses. The motion capture system was used as trigger for synchronization with the force recordings.
Statistical Analysis
The Shapiro-Wilk test was applied to evaluate whether data resembled a normal distribution. The performed tests revealed that all variables, except vertical displacement, were not normally distributed. Thus to not normally distributed data, log10 transformations were applied for data to resemble a normal distribution (Bland & Altman, Citation1996). A student’s paired two-tailed t-test was used to evaluate the difference in tapping rate from the first bout to the second bout in the baseline tapping session. For this particular evaluation, all participants in the study were included (i.e., n = 88). A two-way repeated measures mixed analysis of variance (ANOVA) was performed to evaluate the effects of tapping session (baseline, 20 s, 60 s, 120 s, and 180 s) and group (active and passive). Repeated measures were performed across the five sessions. Thus, differences between tapping sessions within groups were evaluated as a within-subjects factor. Differences between groups performing active tapping and passive tapping were evaluated as a between-subjects factor. In the event that significant main effects or interactions were identified, post hoc pairwise comparisons with Bonferroni correction were performed. To additionally scrutinize the effect of session on freely chosen tapping rate, a similar two-way repeated measures mixed ANOVA was performed. This time, however, data on tapping rates was constituted of the first 20 s of the first bout in the baseline tapping session and the first 20 s in the second bout in tapping sessions including A20, A60, A120, A180, P20, P60, P120, and P180. A student’s paired two-tailed t-test was used to evaluate any within-session difference in tapping rate from the first 20 s of the first bout to the first 20 s of the second bout in all tapping sessions including active tapping as priming (A20, A60, A120, and A180). A one-way repeated measures ANOVA was performed to evaluate a potential carryover effect of tapping session on tapping rate, vertical displacement, and peak force. For this analysis, average values of absolute tapping rate, vertical displacement, and peak force across the first 20 s of the first tapping bout from all test sessions for the active group were used. Data were pooled according to the performed tapping sessions in a time-wise chronological order and used as a within-subjects factor. A post hoc pairwise comparison using Bonferroni correction was performed to identify significant simple effects. Between-day test–retest reliability of the freely chosen finger tapping rate was analyzed by comparing tapping rates from the first bout in the baseline tapping session and in the bout of A180 by intraclass correlation coefficient (ICC), using a Two-way Mixed model for absolute agreement (ICC(3,1)). The statistical analyses were performed using IBM SPSS 25.0 (SPSS Inc., Chicago, IL, USA). Data are presented as average ± SD, unless otherwise indicated. p < 0.05 was considered statistically significant.
Results
Sixty-three participants showed RBRE from the first to the second bout in the baseline tapping session (25 men, 38 women, 1.74 ± 0.10 m, 72.8 ± 12.3 kg, 26.2 ± 5.8 years; 55 right-handed and 8 left-handed). Based on the balancing, a total of 31 participants were allocated to the group in which active tapping was applied as priming and a total of 32 participants were allocated to the group in which passive tapping was applied as priming. Three participants, who were selected for further participation in the experiment, withdrew their participation from the study (n = 2 from the active group; n = 1 from the passive group). Furthermore, two participants did not perform the passive tapping bouts as demonstrated and were consequently excluded prior to the data analysis. In addition, data from one participant from the active group was lost due to a technical error during data recording. In total, data from 28 and 29 participants in the active and passive group, respectively, were analyzed. A Shapiro-Wilk test was performed on the differences from the first bout to the second bout. The test showed that for the baseline tapping session, tapping rate (p = 0.013), peak force (p = 0.002), time to peak force (p < 0.001), and duration of finger contact phase (p < 0.001) were not normally distributed.
Tapping Rate
For all participants (n = 88), the tapping rate amounted to 161.4 ± 52.2 taps min−1 in the first bout and 174.2 ± 57.7 taps min−1 in the second bout (p < 0.001) in the baseline tapping session.
For the selected participants, the main results of the two-way repeated measures mixed ANOVA of tapping rate, vertical displacement, and peak force are presented in . The tapping rates from the first bout in the baseline tapping session and second bouts in the test sessions containing A20, A60, A120, A180, P20, P60, P120, and P180 are depicted in . The post hoc analysis revealed that the tapping rate was increased from the first bout in the baseline tapping session to the second bout in sessions applying 20 s priming (11.0 ± 15.4%, p < 0.001), 60 s priming (12.4 ± 15.6%, p < 0.001), 120 s priming (12.6 ± 14.1%, p < 0.001), and 180 s priming (13.7 ± 13.0%, p < 0.001), respectively.
Figure 3. Average freely chosen tapping rates (± SD). The solid line represents data from the first bout in the baseline tapping session (BTS) and second bouts in the sessions, which included active tapping as priming (A20, A60, A120, and A180). The broken line represents data from the first bout in BTS and second bouts in sessions, which included passive tapping as priming (P20, P60, P120, and P180). *Different from the first bout in the BTS (p < 0.001). For clearness, SD bars are only shown in one direction.
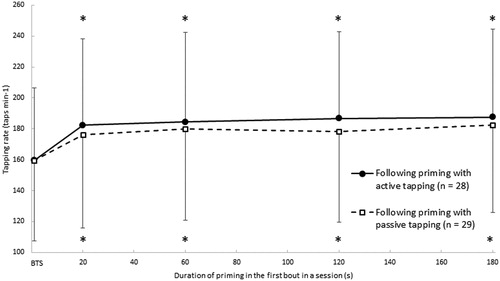
TABLE 1. Results of the two-way repeated measures mixed ANOVA of tapping rate, vertical displacement, peak force, time to peak force, and duration of finger contact phase. Bold text indicates statistical significance.
The additional analysis of the effect of session on the average values of tapping rate across the first 20 s of the first tapping bout in the baseline tapping session and the first 20 s of the second bout in all other test sessions showed the following. A two-way repeated measures mixed ANOVA of tapping rate revealed a significant main effect of Session (F = 24.019, p < 0.001, ƞp2 = 0.304). No main effect was found for Group (F = 0.144, p < 0.705, ƞp2 = 0.003) as well as for Session × Group interaction (F = 0.233, p < 0.878, ƞp2 = 0.004). The post hoc analysis revealed that the freely chosen tapping rate was increased from the first 20 s of the first bout in the baseline tapping session to the first 20 s in the second bout in sessions applying 20 s priming (13.1 ± 17.1%, p < 0.001), 60 s priming (15.0 ± 16.7%, p < 0.001), 120 s priming (14.5 ± 16.6%, p < 0.001), and 180 s priming (16.7 ± 15.5%, p < 0.001), respectively.
For the selected participants who performed active tapping as priming, the freely chosen tapping rate was increased from the first 20 s of the first bout to the first 20 s of the second bout in tapping sessions including A20 (2.9 ± 0.8%, p = 0.035), A60 (3.5 ± 3.4%, p = 0.025), A120 (6.4 ± 1.1%, p < 0.001), and A180 (8.0 ± 1.5%, p < 0.001), respectively.
Peak Force
Absolute values of peak force are presented in , along with vertical displacement, time to peak force, and duration of finger contact phase. The post hoc analysis revealed that the peak force decreased (on average 7.9 ± 12.2%) from the first bout in the baseline tapping session to the second bout in the session applying 180 s priming (p = 0.007).
TABLE 2. Data for both groups of participants from the first bout in the baseline tapping session (BTS) and from the second bouts in sessions including active tapping as priming (A20, A60, A120, and A180) as well as in sessions including passive tapping as priming (P20, P60, P120, and P180). Values are presented as average ± SD.
Examination of Carryover Effect
To assess a potential carryover effect, average values of absolute tapping rate, vertical displacement, and peak force across the first 20 s of the first tapping bout from all test sessions for the active group were analyzed. A one-way repeated measures ANOVA on the tapping rate was significant (F = 8.679, p < 0.001, ƞp2 = 0.243). The post hoc analysis revealed a tendency toward an increased tapping rate between the first bout in the baseline tapping session and the first bout in the second tapping session (p = 0.063). No differences were found between the first bout in the second tapping session and the first bout in the third tapping session (p = 1.000), between the first bout in the third tapping session and the first bout in the fourth tapping session (p = 0.275), and between the first bout in the fourth tapping session and the first bout in the fifth tapping session (p = 1.000). A one-way repeated measures ANOVA on vertical displacement was not significant (F = 1.568, p = 0.211, ƞp2 = 0.059). A one-way repeated measures ANOVA on peak force was not significant (F = 0.170, p = 0.912, ƞp2 = 0.006).
Test-Retest Reliability
The between-day reliability of the freely chosen tapping rate in the present study was high (i.e., ICC(3,1) > 0.80). Thus, ICC(3,1) was 0.85 (p < 0.001) for the freely chosen tapping rate in the first bout in the baseline tapping session versus the tapping rate in the A180 tapping bout.
Discussion
The present study revealed that the phenomenon of RBRE occurred when considering all participants in the study (i.e. n = 88), i.e. before making a selecting of participants for further participation in the study. This finding supported previously reported findings (Emanuelsen et al., Citation2018; Hansen et al., Citation2015; Mora-Jensen et al., Citation2017). For selected individuals, i.e. only those who showed RBRE in a baseline session, priming with active or passive tapping for a range of durations between 20 and 180 s resulted in an enhanced tapping rate. It was in contrast to our expectation that merely 20 s of tapping elicited an enhanced tapping rate.
For the group performing active tapping as priming in the first bout (i.e. by performing A20, A60, A120, and A180), the freely chosen tapping rate was higher in the second bout in all tapping sessions compared with the first bout in the baseline tapping session. In the present study, the relative magnitude of rate enhancement from the first bout in the baseline tapping session to the second bout in the test session containing A180 was on average 17.6%. For comparison, this rate enhancement was higher than a previously reported RBRE of 12.9% from Emanuelsen et al. (Citation2018). It is possible that the discrepancy can be explained by differences between the group samples of participants. Of note, approximately 28% of the gross group of participants in the present study did not elicit RBRE. This is comparable to previous reports of 33% (Mora-Jensen et al., Citation2017) and 36% (Emanuelsen et al., Citation2018). The average increases in tapping rate from the first bout in the baseline tapping session to the second bout in the test sessions containing A20, A60, and A120 ranged from 14.2% to 17.0%. The differences in the relative rate enhancement following A20, A60, and A120 were not different from the difference in the relative rate enhancement following A180. These results show that elicitation of RBRE in the present study occurred independently of the duration of the first bout performed at a freely chosen tapping rate.
It was in contrast to our expectation, that rate enhancement occurred in the test session containing A20. Prior to the study, pilot test data were collected from 18 individuals, where RBRE was not elicited following 30 s priming of active tapping. However, the reason for this discrepancy could be that individuals performing the pilot test were not selected based on showing RBRE, as it was done in the present study. Another possibility is that the sample size of 18 individuals in the pilot study resulted in low statistical power with a type II error as a consequence. In other words, it indeed appears that individuals susceptible to RBRE merely require 20 s or even less time of priming to elicit rate enhancement. It has previously been speculated that RBRE could be the result of an increased net excitability of the nervous system, either 1) of the spinal CPG itself, 2) of supraspinal centers, or 3) by a combination of spinal and supraspinal mechanisms (Emanuelsen et al., Citation2018; Hansen et al., Citation2015). For interpretation of the present results from the group performing active tapping, in relation to the abovementioned three possibilities, mechanisms regulating elicitation of RBRE could be further speculated upon. It has previously been reported that increased supraspinal excitability, but not spinal motoneurone excitability, is present prior to the rhythmic movement of arm cycling when compared to rest (Power & Copithorne, Citation2013). The finding by Power and Copithorne (Citation2013) was suggested to reflect that supraspinal strategies are used to prime the motor system prior to the movement, before spinally located CPG’s assume the control for the regulation of rhythmic movement. Thus, it seems possible that contribution from both spinal and supraspinal mechanisms for the regulation of rhythmic movement, can occur separately in humans. Additionally, it has previously been reported that during repetitive activation of the CPG of the feeding program in Aplysia, effects of modulators become cumulative and leads to a progressive alteration in neuron activity, which results in enhancement of the feeding movement (Cropper et al., Citation2014, Citation2017). Furthermore, it has been reported that an increase in cycle rate of the feeding motor program in Aplysia, controlled by a CPG, is induced by 30 s of electrical stimulation of cerebral-buccal-interneurons, resulting in short-term synaptic enhancement persisting for approximately 2 min (Sánchez & Kirk, Citation2000, Citation2002). Summarizing the abovementioned findings, it appears possible that the observed elicitation of rate enhancement after 20 s of priming in the first tapping bout, could be a result of an increased net excitability of the nervous system from either a spinal, a supraspinal, or a combination of spinal and supraspinal contribution. Of note, the present result generates the obvious question of actually how short a duration of finger tapping, which is required for the elicitation of RBRE?
For the group performing passive tapping as priming in the first bout (i.e. by performing P20, P60, P120, and P180), the freely chosen tapping rate was also higher in the second bout for all tapping sessions compared with the first bout in the baseline tapping session. In the present study, the relative magnitude of rate enhancement from the first bout in the baseline tapping session to the second bout in the test session containing P180 was on average 14.3%. This rate enhancement was higher than a previously reported RBRE magnitude of on average 9.9% (Emanuelsen et al., Citation2018). The equally higher reports of tapping rate enhancement from both the active and passive groups compared to Emanuelsen et al. (Citation2018) could possibly be a reflection of a gross group of participants being more responsive toward elicitation of the phenomenon of RBRE. Also, the increase in tapping rate from the first bout in the baseline tapping session to the second bout in test sessions containing P20, P60, and P120 ranged from 10.5% to 12.8%. The differences in the relative rate enhancement following P20, P60, and P120 were not different from the difference in the relative rate enhancement in P180. These results show that elicitation of rate enhancement in the present study occurred independently of the duration of the first bout of imposed passive tapping. Furthermore, the similar results for the two groups in the present study might reflect that similar mechanisms account for elicitation of RBRE after imposed passive and active tapping. We have previously suggested that increased net excitability of spinal CPG’s and/or involved supraspinal centers, could be caused primarily by peripheral afferent feedback (Emanuelsen et al., Citation2018). The present results further supports this notion as elicitation of rate enhancement occurred during all test sessions including passive tapping as priming. Although the present results do not provide further evidence of differentiation between spinal and supraspinal mechanisms on elicitation of RBRE, possible explanations could be speculated upon. Altered excitability of spinal CPG’s has been shown through pharmacological neuromodulation (Chapman & Sillar, Citation2007; Katz & Harris-Warrick, Citation1990), and electrical stimulation of afferents (Edgerton et al., Citation2008, Etlin et al., Citation2010; Finkel et al., Citation2014) in animals. In addition, spinal cord stimulation in combination with pharmacological neuromodulation in humans results in excitation of the spinal circuitry (Angeli et al., Citation2014; Gad et al., Citation2017). Also, it has been argued that increased corticospinal excitability could be induced by passive movement-associated afferent input (Nakagawa et al., Citation2017). In addition, passive movements, compared to rest, have been reported to show activation of cortical areas involved in motor control (Carel et al., Citation2000). Furthermore, altered cortical activation has been suggested to be induced by input of afferent feedback accompanying passive movements (Reddy et al., Citation2001). Thus, it is possible that elicitation of rate enhancement after passive movements, was a result of a combination of spinal and supraspinal mechanisms. This is in line with the proposed explanation for the group performing active tapping as priming. Elicitation of rate enhancement following passive tapping possibly points toward afferent feedback as the primary contributor for an increased net excitability in the nervous system. For further clarification of possibly neural mechanisms involved in elicitation of RBRE, studies applying techniques such as e.g. magnetic resonance spectroscopy (Wyss et al., Citation2016) can perhaps be performed in the future.
We have previously investigated the within-bout change (first 30 s compared to last 30 s in 3 min bouts) in freely chosen tapping rate and found it to amount to on average 3%-6% (Hansen et al., Citation2015). Moreover, we have previously investigated the within-bout change in 3 min tapping bouts (using three 8-s epochs from the start (0-8 s), mid (86-94 s), and end of the bout (172-180 s)). We found that the tapping rate increased from start to mid (on average 4.6%), from start to end (on average 6.7%), and from mid to end (on average 2.2%) (Emanuelsen et al., Citation2019). To additionally scrutinize the inter-session effects on rate enhancement, an additional analysis on freely chosen tapping rate restricted to the first 20 s of the tapping bouts was performed. Similar results to the analysis of the 180 s tapping bouts were found. These findings suggest that rate enhancement is present early in the 180 s tapping bouts. Moreover, RBRE was documented to occur within sessions for the selected participants who performed active tapping as priming (i.e. A20, A60, A120, and A180). The magnitude of RBRE as a result of active priming ranged from 2.9% to 8.0%. Of note is that these results were calculated for the initial 20 s of the tapping bouts investigated. And this can explain the lower values than the rate enhancement calculated across the entire 180 s of the bouts.
It has previously been reported that the vertical displacement of the index finger is reduced during RBRE (Emanuelsen et al., Citation2018; Mora-Jensen et al., Citation2017). However, the present study did not reveal statistically significant changes in the vertical displacement of the index finger from the first bout in the baseline tapping session to any of the second bouts in the test sessions. The reason for this is not obvious, although the involvement of different participants could be a possible explanation. The tapping force was reduced from the first bout in the baseline tapping session to the second bout in the test sessions containing 180 s of priming. This was in line with previous reports (Emanuelsen et al., Citation2018). However, no statistically significant changes in tapping force were observed from the first bout in the baseline tapping session to the second bout in the test sessions containing 20, 60, and 120 s of priming. Although not obvious, it could be that this was due to type II errors. Thus, a main effect of session on tapping force was found, and as mentioned the post hoc analysis revealed that only 180 s of tapping was significant, in line with Emanuelsen et al. (Citation2018). However, test sessions containing 20 and 60 s of tapping showed tendencies for a reduced tapping force, whereas the test session containing 120 s of tapping was not different.
The analysis of a possible carryover effect revealed that the freely chosen tapping rate performed in each of the first tapping bouts in all tapping sessions for the active group, did not change during the experiment. However, it should be noted for completeness that there was a tendency for the freely chosen tapping rate to increase from the first bout in the baseline tapping session to the first bout in the second test session. It has previously been reported that a two-week washout period is sufficient for the freely chosen tapping rate to return to baseline (Hansen & Ohnstad, Citation2008). This was further supported by Hansen et al. (Citation2015), who reported an ICC of 0.94 from a between-day reliability test comparing the freely chosen tapping rate from an initial bout in a session and comparable data obtained 16 ± 4 days later. A similar between-day reliability test was performed in the present study, replicating a high relative reliability (ICC(3,1) of 0.85). Still for completeness, it should be noted that the experimental designs in Hansen and Ohnstad (Citation2008) and Hansen et al. (Citation2015) did not apply a selection of participants, as in the present study.
A few limitations and strengths of the present study should be considered. During the passive tapping bout, the amount of possible voluntary muscle activation of the involved flexor and extensor muscles were not quantified as previously done (Emanuelsen et al., Citation2018). In the study by Emanuelsen et al. (Citation2018), we reported the amount of voluntary muscle activation (in terms of sEMG activity) to be inconsiderable, when performing this type of passive tapping. Besides, participants in the present study had a more comprehensive familiarization to passive tapping (approximately 5-10 min) than the reported familiarization period in Emanuelsen et al. (Citation2018) (< 5 min). Thus, they were better prepared to perform the tapping bout in the intended passive manner. In an attempt to evaluate the possible amount of voluntary muscle activation during the passive tapping bouts, the participants were first assessed subjectively through visual inspection of the interaction between the participant and the machine. Muscle activation tended to decrease the amplitude of the imposed movement. Alternatively, the finger lost contact with the rocker arm of the machine. In order to make an objective evaluation of unintended voluntary muscle activation, it was defined that participants who needed more than 2 s of adaptation to the passive tapping were excluded. This criterion of 2 s corresponded to more than 10% of the duration of A20 and more than 1% of A180. As a result of this criterion, data from two participants were excluded from the data set. Furthermore, it has recently been reported that corticospinal excitability is increased when paying attention to passive finger movements (Tsuiki et al., Citation2019). In the present study, the participants were neither instructed to pay attention, nor not to pay attention, to the passive movement of the finger. Rather, they were instructed to relax in order to perform the passive tapping. However, it cannot be ruled out that some participants performed the passive tapping with attention and that this possibly contributed to an increased corticospinal excitability. The lack of concurrent changes in tapping rate and tapping force and/or vertical displacement could be attributed to the fact that the results are based on group averages that are calculated on the basis of variables, which in turn are averages of variables from series of taps. In this process it could be that information is lost, as a given increase in tapping rate can be obtained by different combinations of tapping force and displacement. Future studies could investigate the potential effect of warm up on elicitation of RBRE. This could e.g. be done by passively warming up the involved muscles.
Conclusion
The main finding of the present study was that priming, in form of active or passive tapping for a range of durations between 20 and 180 s, elicited rate enhancement. The increased tapping rate following priming is suggested to be a result of increased net excitability of the nervous system.
AUTHOR CONTRIBUTIONS
EH and AE developed the initial idea for the study. All authors contributed to the planning of the study design. AE performed the data collection. AE and MV performed most of the data analyses assisted by PM and EH. All authors contributed to the interpretation of the results. AE completed the first draft of the manuscript. All authors participated in revising of drafts to finalize the manuscript.
Acknowledgments
The participants are thanked for their cooperation.
DISCLOSURE STATEMENT
The present research was conducted without any financial or commercial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Angeli, C.A., Edgerton, V.R., Gerasimenko, Y.P. and Harkema, S.J., 2014. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain, 137(Pt 5), 1394–1409. https://doi.org/10.1093/brain/awu038
- Bland, M.J. and Altman, D.J., 1996. Transforming data. BMJ, 312, 770. https://doi.org/10.1136/bmj.312.7033.770
- Burke, R. E., Degtyarenko, A. M. and Simon, E. S., 2001. Patterns of locomotor drive to motoneurons and last-order interneurons: Clues to the structure of the CPG. Journal of Neurophysiology, 86(1), 447–462.
- Carel, C., Loubinoux, I., Boulanouar, K., Manelfe, C., Rascol, O., Celsis, P. and Chollet, F., 2000. Neural substrate for the effects of passive training on sensorimotor cortical representation: A study with functional magnetic resonance imaging in healthy subjects. Journal of Cerebral Blood Flow and Metabolism, 20(3), 478–484. https://doi.org/10.1097/00004647-200003000-00006
- Chapman, R.J. and Sillar, K.T., 2007. Modulation of a spinal locomotor network by metabotropic glutamate receptors. European Journal of Neuroscience, 26, 2257–2268. https://doi.org/10.1111/j.1460-9568.2007.05817.x
- Collyer, C. E., Broadbent, H. A. and Church, R. M., 1992. Categorical time production: Evidence for discrete timing in motor control. Perception & Psychophysics, 51(2), 134–144. https://doi.org/10.3758/bf03212238
- Cropper, E.C., Friedman, A.K., Jing, J., Perkins, M.H. and Weiss, K.R., 2014. Neuromodulation as a mechanism for the induction of repetition priming. Current Opinion in Neurobiology, 29(12), 33–38. https://doi.org/10.1016/j.conb.2014.04.011
- Cropper, E.C., Jing, J., Perkins, M.H. and Weiss, K.R., 2017. Use of the Aplysia feeding network to study repetition priming of an episodic behavior. Journal of Neurophysiology, 118(3), 1861–1870. https://doi.org/10.1016/j.brainresrev.2009.08.002
- Dietz, V., 2003. Spinal cord pattern generators for locomotion. Clinical Neurophysiology, 114(8), 1379–1389. https://doi.org/10.1016/S1388-2457(03)00120-2
- Dimitrijevic, M.R., Gerasimenko, Y. and Pinter, M.M., 1998. Evidence for a spinal central pattern generator in Humansa. Annals of the New York Academy of Sciences, 860(1), 360–376. https://doi.org/10.1111/j.1749-6632.1998.tb09062.x
- Edgerton, V.R., Courtine, G., Gerasimenko, Y.P., Lavrov, I., Ichiyama, R.M., Fong, A.J., Cai, L.L., Otoshi, C.K., Tillakaratne, N.J.K., Burdick, J.W. and Roy, R.R., 2008. Training locomotor networks. Brain Research Reviews, 57(1), 241–254. https://doi.org/10.1016/j.brainresrev.2007.09.002
- Emanuelsen, A., Madeleine, P., Voigt, M and Hansen, E.A., 2019. Motor variability in elicited repeated bout rate enhancement is associated with higher sample entropy. Human Movement Science, 68:102520. https://doi.org/10.1016/j.humov.2019.102520
- Emanuelsen, A., Voigt, M., Madeleine, P., Kjaer, P., Dam, S., Koefoed, N. and Hansen, E.A., 2018. Repeated Bout Rate Enhancement Is Elicited by Various Forms of Finger Tapping. Frontiers in Neuroscience, 12:526. https://doi.org/10.3389/fnins.2018.00526
- Etlin, A., Blivis, D., Ben-Zwi, M. and Lev-Tov, A., 2010. Long and short multifunicular projections of sacral neurons are activated by sensory input to produce locomotor activity in the absence of supraspinal control. Journal of Neuroscience, 30(31), 10324–10336. https://doi.org/10.1523/JNEUROSCI.1208-10.2010
- Finkel, E., Etlin, A., Cherniak, M., Mor, Y., Lev‐Tov, A. and Anglister, L., 2014. Neuroanatomical basis for cholinergic modulation of locomotor networks by sacral relay neurons with ascending lumbar projections. Journal of Comparative Neurology, 522(15), 1379–3455. https://doi.org/10.1002/cne.23613
- Frigon, A., 2017. The neural control of interlimb coordination during mammalian locomotion. Journal of Neurophysiology, 117(6), 2224–2241. https://doi.org/10.1152/jn.00978.2016
- Gad, P., Gerasimenko, Y., Zdunowski, S., Turner, A., Sayenko, D., Lu, D.C. and Edgerton, V.R., 2017. Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Frontiers in Neuroscience, 11:333. https://doi.org/10.3389/fnins.2017.00333
- Goulding, M., 2009. Circuits controlling vertebrate locomotion: moving in a new direction. Nature Reviews. Neuroscience, 10(7), 507–518. https://doi.org/10.1038/nrn2608
- Grillner, S., 2009. Pattern generation. Encyclopedia of Neuroscience, 7, 487–494. https://doi.org/10.1016/B978-008045046-9.01341-3
- Hansen, E.A., Ebbesen, B.D., Dalsgaard, A., Mora-Jensen, M.H. and Rasmussen, J., 2015. Freely chosen index finger tapping frequency is increased in repeated bouts of tapping. Journal of Motor Behavior, 47(6), 490–496. https://doi.org/10.1080/00222895.2015.1015675
- Hansen, E.A. and Ohnstad, A.E., 2008. Evidence for freely chosen pedalling rate during submaximal cycling to be a robust innate voluntary motor rhythm. Experimental Brain Research, 186(3), 365–373. https://doi.org/10.1007/s00221-007-1240-5
- Katz, P.S. and Harris-Warrick, R.M., 1990. Neuromodulation of the crab pyloric central pattern generator by serotonergic/cholinergic proprioceptive afferents. Journal of Neuroscience, 10(5), 1495–1512. https://doi.org/10.1523/JNEUROSCI.10-05-01495.1990
- Klarner, T. and Zehr, E.P., 2018. Sherlock Holmes and the curious case of the human locomotor central pattern generator. Journal of Neurophysiology, 120(1), 53–77. https://doi.org/10.1152/jn.00554.2017
- MacKay-Lyons, M., 2002. Central Pattern Generation of Locomotion: A Review of the Evidence. Physical Therapy, 82(1), 69–83. https://doi.org/10.1093/ptj/82.1.69
- Madison, G., 2001. Variability in isochronous tapping: Higher order dependencies as a function of intertap interval. Journal of Experimental Psychology: Human Perception and Performance, 27(2), 411–422 https://doi.org/10.1037/0096-1523.27.2.411
- Mora-Jensen, M.H., Madeleine, P. and Hansen, E.A., 2017. Vertical finger displacement is reduced in index finger tapping during repeated bout rate enhancement. Motor Control, 21(4), 457–467. https://doi.org/10.1123/mc.2016-0037
- Moussay, S., Dosseville, F., Gauthier, A., Larue, J., Sesboüe, B. and Davenne, D., 2002. Circadian rhythms during cycling exercise and finger-tapping task. Chronobiology International, 19(6), 1137–1149. https://doi.org/10.1081/CBI-120015966
- Nadim, F. and Bucher, D., 2014. Neuromodulation of neurons and synapses. Current Opinion in Neurobiology, 29:48–56. https://doi.org/10.1016/j.conb.2014.05.003
- Nakagawa, M., Sasaki, R., Tsuiki, S., Miyaguchi, S., Kojima, S., Saito, K., Inukai, Y. and Onishi, H., 2017. Effects of Passive Finger Movement on Cortical Excitability. Frontiers in Human Neuroscience, 11:216. https://doi.org/10.3389/fnhum.2017.00216
- Power, K.E. and Copithorne, D.B., 2013. Increased corticospinal excitability prior to arm cycling is due to enhanced supraspinal but not spinal motoneurone excitability. Applied Physiology, Nutrition, and Metabolism, 38(11), 1154–1161. https://doi.org/10.1139/apnm-2013-0084
- Prochazka, A. and Ellaway, P., 2012. Sensory systems in the control of movement. Comprehensive Physiology, 2(4), 2615–2627. https://doi.org/10.1002/cphy.c100086
- Reddy, H., Floyer, A., Donaghy, M. and Matthews, P., 2001. Altered cortical activation with finger movement after peripheral denervation: comparison of active and passive tasks. Experimental Brain Research, 138(4), 484–491. https://doi.org/10.1007/s002210100732
- Sakamoto, M., Tazoe, T., Nakajima, T., Endoh, T., Shiozawa, S. and Komiyama, T., 2007. Voluntary changes in leg cadence modulate arm cadence during simultaneous arm and leg cycling. Experimental Brain Research, 176(1), 188–192. https://doi.org/10.1007/s00221-006-0742-x
- Sánchez, J.A.D. and Kirk, M.D., 2000. Short-term synaptic enhancement modulates ingestion motor programs of aplysia. Journal of Neuroscience, 20:RC85 (1-7). https://doi.org/10.1523/JNEUROSCI.20-14-j0004.2000
- Sánchez, J.A.D. and Kirk, M.D., 2002. Ingestion motor programs of Aplysia are modulated by short-term synaptic enhancement in cerebral-buccal interneuron pathways. Invert. Neurosci , 4(4), 199–212. https://doi.org/10.1007/s10158-002-0021-x
- Sardroodian, M., Madeleine, P., Mora-Jensen, M.H. and Hansen, E.A., 2016. Characteristics of finger tapping are not affected by heavy strength training. Journal of Motor Behavior, 48(3), 256–263. https://doi.org/10.1080/00222895.2015.1089832
- Schlinger, H.D.J., 2015. Behavior analysis and the good life. Philosophy, Psychiatry, and Psychology, 22(4), 267–270. https://doi.org/10.1353/ppp.2015.0052
- Shima, K., Tamura, Y., Tsuji, T., Kandori, A. and Sakoda, S., 2011. A CPG synergy model for evaluation of human finger tapping movements. 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, 4443–4448. https://doi.org/10.1109/IEMBS.2011.6091102
- Siniscalchi, M.J., Cropper, E.C., Jing, J. and Weiss, K.R., 2016. Repetition priming of motor activity mediated by a central pattern generator: The importance of extrinsic vs. intrinsic program initiators. Journal of Neurophysiology, 116(4), 1821–1830. https://doi.org/10.1152/jn.00365.2016
- Stang, J., Wiig, H., Hermansen, M. and Hansen, E.A., 2016. Voluntary movement frequencies in submaximal one-and two-legged knee extension exercise and pedaling. Frontiers in Human Neuroscience, 10:36. https://doi.org/10.3389/fnhum.2016.00036
- Tsuiki, S., Sasaki, R., Pham, M.V., Miyaguchi, S., Kojima, S., Saito, K., Inukai, Y., Otsuru, N. and Onishi, H., 2019. Repetitive passive movement modulates corticospinal excitability: effect of movement and rest cycles and subject attention. Frontiers in behavioral neuroscience, 13:38. https://doi.org/10.3389/fnbeh.2019.00038
- Wyss, P.O., Hock, A. and Kollias, S., 2016. the application of human spinal cord magnetic resonance spectroscopy to clinical studies: A review. Seminars in Ultrasound, CT, and MRI, 38(2), 153–162. https://doi.org/10.1053/j.sult.2016.07.005
- Zehr, E.P., 2005. Neural control of rhythmic human movement: the common core hypothesis. Exercise and Sport Sciences Reviews, 33(1), 54–60.
- Zehr, E.P., Carroll, T.J., Chua, R., Collins, D.F., Frigon, A., Haridas, C., Hundza, S.R. and Thompson, A.K., 2004. Possible contributions of CPG activity to the control of rhythmic human arm movement. Canadian Journal of Physiology and Pharmacology, 82(8), 556–568. https://doi.org/10.1139/y04-056
- Zehr, E.P. and Duysens, J.E.J., 2004. Regulation of arm and leg movement during human locomotion. Neuroscientist, 10(4), 347–361. https://doi.org/10.1177/1073858404264680
