 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Zr solubility in the presence of gluconic acid (GLU) and isosaccharinic acid (ISA) was investigated as a function of hydrogen ion concentration (pHc) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pHc and GLU concentration suggested the existence of Zr(OH)4(GLU)22− in the neutral pH region and Zr(OH)4(GLU)(GLU-H)3− in the alkaline pH region above pHc 10 as the dominant species in the presence of 10−3–10−1 mol/dm3 (M) GLU. In the presence of ISA, the dominant species Zr(OH)4(ISA)22− and Zr(OH)4(ISA)(ISA-H)3− were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH)4(am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least-squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
1. Introduction
For the safety assessment of radioactive waste disposal, it is very important to predict the solubility limit of radionuclides under relevant conditions. Isosaccharinic acid (ISA), a degradation product of cellulose found in low- and intermediate-level radioactive waste, is known to form strong complexes with radionuclides [Citation1–3]. Gluconic acid (GLU), an organic compound used as a cement additive, has been found to form stable complexes with metal ions [Citation4–6]. GLU is sometimes considered to be an analog of ISA due to its similarity in structure. Under repository conditions, in the presence of the aforementioned organic acids, the solubility of radionuclides could potentially be enhanced, which could affect the safety assessment of radioactive waste disposal. Since the solubility and complexation behavior of tetravalent actinide (An(IV)) is primarily controlled by the solubility of the sparingly soluble solid phase amorphous hydroxide (An(OH)4(am)) and concentration of complexing agent, respectively, it is necessary to quantify the impact of the complexation ability of the organic acids on the solubility of An(OH)4(am).
Interaction of organic acids with tetravalent actinides relevant to geological disposal has been reviewed as part of the Nuclear Energy Agency Thermochemical Database Project (NEA-TDB) [Citation7]. Although several reports have investigated the interaction of ISA with tetravalent actinides, the number of reported values of thermodynamic constants such as complex formation constants were considerably limited [Citation7]. To achieve a reliable prediction of solubility in the presence of organic acids, the complexation ability of ISA and GLU toward An(IV) has been intensively investigated recently. Rai et al. conducted a solubility experiment on Np(IV) in the presence of ISA across a wide range of pH values and proposed the ternary complexes Np(OH)3(ISA)(aq), Np(OH)3(ISA)2−, Np(OH)4(ISA)−, and Np(OH)4(ISA)22− [Citation8]. Warwick et al. investigated the solubility of U(IV) in the presence of ISA and GLU and suggested the formation of the complexes U(OH)4(ISA)− and U(OH)4(GLU)− [Citation9]. On the other hand, the interaction of Pu(IV) with ISA was examined only in a narrow alkaline pH range [Citation10,Citation11] and complexation behavior in a wide pH range was not sufficiently elucidated. Rai and coworkers intensively researched the solubility of Th(IV) under a wide range of pH and ISA concentrations, based on the complex formation constants of ThOH(ISA)2+, Th(OH)3(ISA)2−, and Th(OH)4(ISA)22− [Citation12]. Gaona et al. carried out a comprehensive review on the complexation of tetravalent actinides with ISA and GLU [Citation13]. Based on the reported and recalculated values of literature data, a systematic trend in the complex formation constants between tetravalent actinides has been discussed using a linear free-energy relationship [Citation13]. However, the literature is not extensive enough and is limited within narrow experimental conditions, which indicates the necessity to investigate this topic further and to determine the thermodynamic constants of gluconate and isosaccharinate complex formation. Furthermore, although important similarities have been observed between the stability of the gluconate and isosaccharinate complexes of tetravalent actinides, a different stoichiometry of isosaccharinate and gluconate complexes has also been reported in the literature [Citation13]. This suggests that the slight difference in the structure of the polyhydroxy carboxylic acids is closely linked to their different complexation abilities. The different properties and mechanisms associated with the stability of the isosaccharinate and gluconate complexes with tetravalent actinides have not been well researched.
In the present study, we focused on the solubility of zirconium in the presence of GLU and ISA. As a tetravalent ion, zirconium is considered to be a chemical analog of tetravalent actinides (such as Th(IV), U(IV), Np(IV), and Pu(IV)), although different chemical characteristics have also been observed [Citation14–16]. Zirconium is a relevant element in the safety assessment of radioactive waste disposal since it has a high yield in uranium fission products and zirconium metal is used as a fuel cladding material in light-water reactors. However, no thermodynamic data on Zr gluconate and isosaccharinate are available. The present study has investigated Zr solubility in the presence of GLU and ISA in a wide range of hydrogen ion concentrations (pHc) and total concentrations of GLU or ISA. Thermodynamic analysis of Zr solubility revealed the dominant soluble complexes and solid phases in the Zr gluconate and isosaccharinate systems. The formation constants of the Zr gluconate and isosaccharinate complexes were determined by the least squares fitting analysis of the solubility data and discussed in comparison with those of the tetravalent actinides.
2. Experimental methods and materials
2.1. Chemicals and analytical methods
All chemicals used were of reagent grade. ZrCl4 was purchased from Sigma-Aldrich to prepare a stock solution of Zr(IV) perchlorate. Deionized purified water (Milli-Q, Millipore) was used in all solution preparations. ZrCl4 was dissolved in purified water and portions of NaOH solution (Wako Pure Chem.) were then added to the zirconium chloride solution to neutralize and precipitate zirconium hydroxide (Zr(OH)4(am)). The precipitate was washed with purified water several times and finally suspended in purified water as a Zr(OH)4(am) stock suspension. Calcium isosaccharinate (Ca(ISA)2) was prepared using the method reported by Whistler and BeMiller from α-lactose (Wako Pure Chem.) [Citation17] and characterized by X-ray diffraction (XRD) by comparing the spectra in literature [Citation8]. Elemental analysis calculated for C12H22O12Ca: C 36.18, H 5.57%; found, C 34.49, H 5.63%. A stock solution of 0.5 mol/dm3 (M) sodium isosaccharinate (NaISA) was then prepared from Ca(ISA)2 using the method described in the literature [Citation12]. A stock solution of 0.5 M sodium gluconate (NaGLU) was prepared by dissolving a reagent from Wako Pure Chem. shows chemical formula of α-isosaccharinic acid and gluconic acid.
A combination glass electrode (9615-10D, Horiba Ltd.) was used to measure the pHc. The reference electrode was filled with 3.6 M NaCl and 0.4 M NaClO4 (Wako Pure Chem.). The electrode was calibrated against standard HCl and NaOH solutions (pHc 1, 2, 3, 11, 12, and 13; Wako Pure Chem.) at ionic strength (I) = 0.5 M by NaCl to correct the experimentally measured pHexp values to the pHc values.
2.2. Solubility experiments
Sample solutions were prepared by an undersaturation approach. Sample solutions in the acidic and neutral pH range (pHc < 8) were prepared and stored under atmospheric conditions and those in the alkaline pH range (pHc > 8) were prepared and stored in an Ar glove box (O2 < 0.1 ppm) at 25 °C. An aliquot of NaGLU stock solution and HCl and/or NaOH solutions were added to polypropylene tubes to prepare sample solutions of a specific pHc and GLU concentration. The ionic strength was fixed at I = 0.5 M by adding appropriate amounts of NaCl. A portion of Zr(OH)4(am) stock suspension was then added to the polypropylene tubes such that the Zr concentration corresponded to 0.01 M if the solid phase was completely dissolved. The pHc of the sample solutions ranged from 4 to 12.5, and the GLU concentration ranged from 10−5 to 10−1 M. The sample solutions containing ISA were prepared in the same manner. The pHc of the sample solution ranged from 2 to 12.5, and the ISA concentration ranged from 10−3 to 10−1 M. The sample tubes were then left for a given aging time up to 16 weeks and sometimes shaken by hand for a few minutes during the aging process. After the given aging time, the pHc value of each sample solution was measured and the supernatant of the sample solution was filtered through ultrafiltration membranes (Microcon, Nominal Molecular Weight Limit (NMWL) 10 kDa, corresponding to pore sizes of approximately 3 nm, Millipore). After filtration, a small amount of nitric acid was added to the filtrate immediately to avoid any sorption of Zr species on the sample tube throughout the solubility experiment. The filtrate was diluted in 0.1 M nitric acid and then measured by inductively coupled plasma mass spectrometry (ICP-MS; ElanDRC II, PerkinElmer) to determine the Zr concentration. The detection limit of Zr was about 10−8.5 M. For each measurement carried out by ICP-MS, the standard error was within 10%, resulting in ±0.1 in log units of Zr concentration. After the solubility measurement, the supernatants of several sample solutions were removed by centrifugation, and the solid phases were dried at room temperature. The solid phases were then investigated by XRD methods (RINT 2000, Rigaku) using a Cu X-ray (λKα = 1.54 Å) within a range of 2θ = 10°−60°.
3. Results and discussion
3.1. Zr solubility in the presence of gluconic acid
shows Zr solubility in the presence of 10−5–10−1 M GLU after ultrafiltration through 10 kDa membranes as a function of pHc, over an aging period of 12–16 weeks. Since the solubility data after 12 and 16 weeks showed similar values, a steady state was considered to be achieved within 12 weeks. Literature data on Zr solubility in the absence of GLU [Citation18–20] were plotted for comparison. In the presence of GLU below 10−3 M, the solubility values were approximately 10−8.5 M, which is the detection limit level of the ICP-MS (indicated by a gray field in the figure). In the presence of GLU above 10−2.5 M, the solubility clearly increased from the solubility of Zr(OH)4(am) or Zr(OH)4(s), depending on the GLU concentration, which indicated the formation of a Zr gluconate complex. For pHc 4–8, the solubility was observed to be independent of pHc, suggesting that four OH− ions are involved in the gluconate complex in this pH region. On the other hand, above pHc 10, the solubility increased with increasing pHc with a slope of approximately 1. The trend in solubility suggests that one additional OH− ion is involved in the reaction between the solid phase and gluconate complex. shows the Zr solubility at pHc 7.8 and 10.6 as a function of the total GLU concentration ([GLU]tot). The slope of Zr solubility against GLU concentration is approximately 2, indicating that two gluconates are involved in the reaction.
Figure 2. Zr solubility in the presence of 10−5–10−1 M gluconic acid (GLU) after ultrafiltration through 10 kDa membranes.
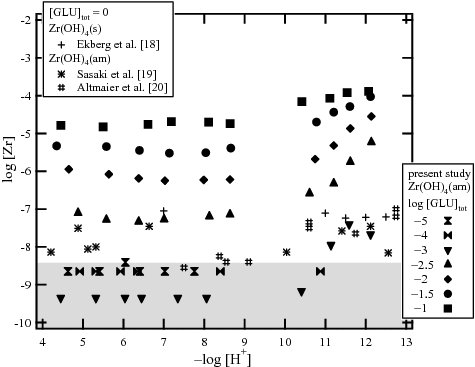
Figure 3. Zr solubility at pHc 7.8 and 10.6 after ultrafiltration through 10 kDa membranes as a function of total GLU concentration ([GLU]tot).
![Figure 3. Zr solubility at pHc 7.8 and 10.6 after ultrafiltration through 10 kDa membranes as a function of total GLU concentration ([GLU]tot).](/cms/asset/acaada53-3aa6-4e91-b410-26ffc81c09a4/tnst_a_1255573_f0003_b.gif)
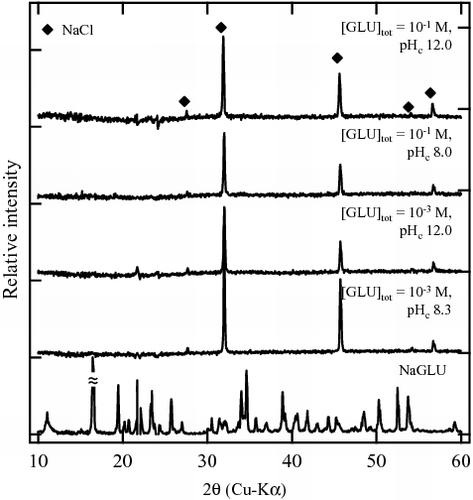
shows the XRD patterns of the Zr solid phases aged in the presence of 10−3 and 10−1 M GLU, together with the reference pattern of NaGLU. In the XRD spectra of the solid phases aged in both 10−3 M and 10−1 M GLU, only three peaks (31.6, 45.4, and 56.4°) corresponding to NaCl(cr) (JCPDS file 050628) used as an electrolyte in the investigated system were found. Since no additional peak was observed, it was considered that the initial Zr(OH)4(am) was not transformed throughout the solubility experiment. It is noted that at pHc 12.1 and 10−1 M GLU concentration, Zr solubility seemed to become saturated at 10−4 M. This may suggest that another solid phase containing gluconate controls the apparent Zr solubility; however, no indication of this was obtained from the XRD patterns ().
3.2. Zr solubility in the presence of ISA
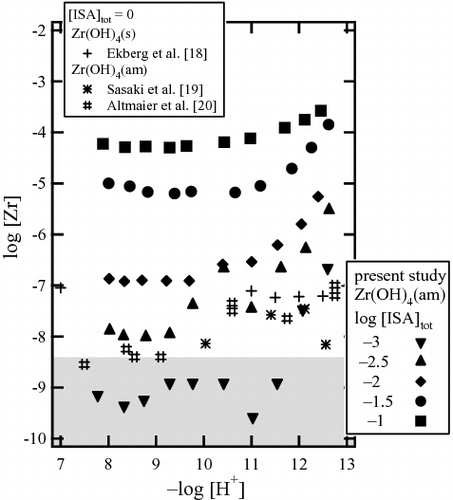
shows Zr solubility after 14 weeks in the presence of 10−3–10−1 M ISA in a pHc range of 8–12.5 after ultrafiltration through 10 kDa membranes. The solubility measurement was repeated after 4, 10, and 14 weeks, and a steady state was confirmed to be achieved within 10 weeks. In the presence of ISA with a concentration higher than 10−2.5 M, Zr solubility was found to be higher in comparison with systems with an absence of ISA, depending on the pHc and ISA concentration [Citation18–20]. Zr solubility seemed to be almost independent of pHc under weakly alkaline pH conditions between pHc 8 and 10, indicating that four OH− ions are involved in the Zr isosaccharinate complex, provided that Zr(OH)4(am) is in the solid phase. In a similar manner to the case of GLU, Zr solubility increased with a slope of approximately 1 above pHc 10, suggesting that one additional OH− was involved in the reaction in this pH region. This may be due to further coordination of an OH− to the Zr isosaccharinate complex or to deprotonation of the hydroxyl group on the main chain of ISA. In , the Zr solubility at pHc 9.3 and 12.1 was shown as a function of the total ISA concentration ([ISA]tot). The slope against the total ISA concentration was approximately 2, indicating that two isosaccharinate molecules were involved in the formation of a Zr isosaccharinate complex. It was noted that no significant peak except that of NaCl(cr) was observed in the XRD patterns of the solid phase aged in the presence of 10−1 M ISA. Meanwhile, Zr solubility in the presence of ISA in the acidic pHc range showed a different tendency from that observed in the presence of GLU. The solubility gradually decreased with increasing pHc, possibly due to the existence of dominant ISA complex with different stoichiometry compared to the GLU complex. Since an anomalous gap was observed between the solubility data in the presence of 10−2 and 10−2.5 M [ISA]tot, detailed results were discussed in the Supplementary material and the solubility data were not included in the following thermodynamic analysis.
3.3. A thermodynamic model of Zr solubility in the presence of gluconic acid
The solid phase aged in the presence of GLU was considered to be Zr(OH)4(am) based on the XRD patterns of the precipitate. Zr solubility was independent of pHc in the pHc range of 4–10 and increased with increasing pHc at pHc > 10 with a slope of 1 (). At a fixed pHc within the neutral to alkaline pH region, the slope of the solubility against GLU concentration was approximately 2 (). Therefore, the equilibrium reactions of the dominant soluble species in the presence of low GLU can be described as:
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
(5)
(5)
(6)
(6)
One of the hydroxyl groups on the alkyl chain of GLU may be deprotonated in the alkaline pH range [Citation13,Citation21,Citation22] to bind the Zr gluconate complex:
(7)
(7)
The formation of Zr(OH)5(GLU)23− and Zr(OH)4(GLU)(GLU-H)3− is compatible with the hydrolysis of Zr and deprotonation of the hydroxyl groups of GLU. Therefore, the difference between the formation of Zr(OH)5(GLU)23− and Zr(OH)4(GLU)(GLU-H)3− does not appear in the solubility experiment and subsequent slope analysis in the present study. We used the chemical formula of Zr(OH)4(GLU)(GLU-H)3− to represent the species in the alkaline pH region, as described below:
(8)
(8)
(9)
(9)
In previous study [Citation23], complexation of zirconium ions by linear alkyl chain dicarboxylic acids from oxalic acid (HOOC–COOH) to glutaric acid (HOOC–(CH2)3–COOH) was investigated. The chelating effect was not found to occur in dicarboxylic acids with alkyl chains longer than that of malonic acid (HOOC–CH2–COOH) due to steric effects inhibiting the formation of a stable complex. The hydrolysis reaction of the tetravalent metal ions surpassed the complexation ability of these dicarboxylic acids in higher pH conditions [Citation23]. In contrast, GLU significantly affected Zr solubility in the neutral and alkaline pH regions. This may indicate that the hydroxyl groups of GLU are involved in the stabilization of the Zr gluconate complex even in the neutral pH region, as proposed for Th gluconate, for which deprotonation of the two hydroxyl groups on the alkyl chain was proposed with the resultant complex Th(OH)2(GLU-2H)− [Citation22].
In addition to the complex formation reactions above, taking the deprotonation of the carboxylic group of GLU [Citation24] and the hydrolysis reactions of Zr [Citation25] into account, Zr solubility as a sum of the concentrations of soluble species ([Zr]) and the total GLU concentration ([GLU]tot) were described as below:
(10)
(10)
(11)
(11)
The complex formation constants of Zr gluconate were then determined by the least squares fitting analysis of the solubility data, as shown in . The fixed and determined parameters in the analysis are summarized in . The fixed parameters were used after correction at I = 0.5 by the specific ion interaction theory (SIT) [Citation26]. Based on the SIT approach, the conditional complex formation constant (β1xy) for Zr(OH)x(GLU)y4−x−y can be described as:(12)
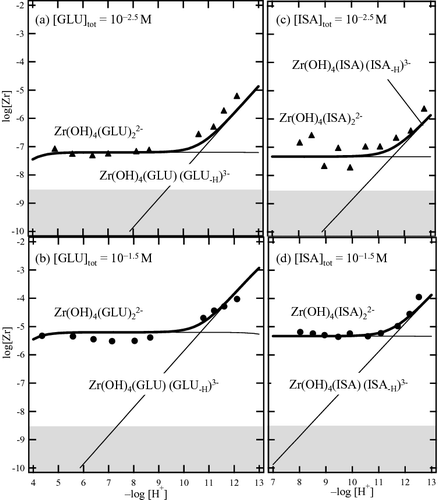
(12) where β°1xy is the complex formation constant at I = 0. The ϵ(Zr(OH)x(GLU)4 − x − yy, Na+),ϵ(Zr4 +, Cl−), ϵ(OH−, Na+),ϵ(GLU−, Na+), and D represent the ion interaction coefficient for each species and the Debye-Hückel term at 25 °C. The ion interaction parameter values for Zr4+, ZrOH3+, Zr(OH)22+, Zr(OH)3+, and OH− were 0.84, 0.55, 0.52, 0.23, and 0.04, respectively [Citation25,Citation27,Citation28]. Similar to the previous study on Zr dicarboxylate [Citation23,Citation29], the ion interaction parameter values of zirconium gluconate were all assumed to be 0, and the value ϵ (CH3COO−, Na+) = 0.08 [Citation26] was used for gluconate due to lack of data in the literature. (a) and (b) represent the solubility curve in the presence of 10−2.5 M and 10−1.5 M GLU and the contributions of the gluconate complexes, respectively, as calculated from the thermodynamic constants in . The solubility values were well described when assuming the proposed complexes.
Table 1. Summary of the complex formation constants and solubility products in the zirconium–gluconate/isosaccharinate systems (I = 0).
Figure 7. Solubility curve and soluble species in the presence of GLU and ISA with a total concentration of 10−2.5 and 10−1.5 M, respectively. The plots show the experimental values and the solid and bold curves represent the gluconate/isosaccharinate complexes and the zirconium solubility, respectively, calculated from the constants shown in
3.4. A thermodynamic model of Zr solubility in the presence of ISA
In the Zr–isosaccharinate system, the initial Zr(OH)4(am) was not transformed in the presence of ISA. Since the dependency of the solubility on pH and ISA concentration is similar to that found in the gluconate system, the same stoichiometry of the isosaccharinate complexes as that of the gluconate complexes is assumed for the Zr–isosaccharinate system:
(13)
(13)
(14)
(14)
(15)
(15)
(16)
(16)
Taking the isosaccharinate complex formation reactions, the deprotonation of the carboxylic group of ISA [Citation12], the lactonization of ISA [Citation12], and the hydrolysis reactions of Zr [Citation25] into account, Zr solubility ([Zr]) and the total ISA concentration ([ISA]tot) can be described as:
(17)
(17)
(18)
(18)
The solubility data in the neutral to alkaline pH range were analyzed to determine the complex formation constants β142 and β152.
The fixed and determined parameter values in the least squares fitting analysis are summarized in , and the solubility curve and the contributions of the isosaccharinate complexes in the presence of 10−2.5 M and 10−1.5 M ISA are shown in , respectively. The fixed parameters were corrected by SIT using the same ion interaction coefficient values as for the gluconate system. In comparison with the complex formation constants of Zr isosaccharinate and gluconate, the β142 value was quite similar, while the β152 value for isosaccharinate was slightly lower than that for gluconate. In the alkaline pH region, one of the hydroxyl groups on the alkyl chain of GLU and ISA may be deprotonated to form the Zr(OH)4(GLU)(GLU-H)3–; and Zr(OH)4(ISA)(ISA-H)3– complexes. The difference in the β152 values may be due to the difference in the deprotonation of the hydroxyl groups of GLU and ISA. In , the complex formation constants of zirconium and tetravalent actinide gluconate and isosaccharinate are summarized. The complex formation constant of β142 for the Zr isosaccharinate complex determined in the present study is close to that of Np(IV) [Citation30]. On the other hand, the stoichiometry of the Zr gluconate complexes proposed in the present study is different from that reported for tetravalent actinide. In the literature, experiments on tetravalent actinide gluconate [Citation4,Citation6,Citation9,Citation22] were mostly conducted at alkaline pH and M(OH)4(GLU)– was proposed as the dominant species, while Zr(OH)4(GLU)22– was proposed in the neutral to weakly alkaline pH range. The difference in the experimental pH range could be as a result of the different stoichiometry of the dominant gluconate complex.
Table 2. Summary of the thermodynamic constants in the zirconium and tetravalent actinide–gluconate/isosaccharinate systems (I = 0).
4. Conclusions
In the present study, the solubility and solid phases of zirconium in the presence of gluconic acid (GLU) and ISA were investigated as a function of the pHc and the total concentrations of GLU and ISA. Zr solubility in the presence of GLU suggested the existence of Zr(OH)4(GLU)22– and Zr(OH)4(GLU)(GLU-H)3– as the dominant species in the pHc range of 4–12.5, and Zr(OH)4(ISA)22– and Zr(OH)4(ISA)(ISA-H)3– were suggested as the dominant species in the presence of ISA. The formation constants and solubility products of the Zr gluconate and isosaccharinate complexes determined in the analysis explained the solubility in the presence of GLU and ISA.
Supplementary_Data.pdf
Download PDF (308.3 KB)Acknowledgements
This work was performed as part of the Project on ‘The project for validating assessment methodology in geological disposal system’ funded by the Agency for Natural Resources and Energy, Ministry of Economy, Trade and Industry of Japan. The authors would like to acknowledge Dr D. Rai for his encouragement and technical discussions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Greenfield BF, Moreton AD, Spindler MW, et al. The effects of the degradation of organic materials in the near field of a radioactive waste repository. Mater Res Soc Symp Proc. 1992;257:299–306.
- Moreton AD. Thermodynamic modelling of the effect of hydroxycarboxylic acids on the solubility of plutonium at high pH. Mater Res Soc Symp Proc. 1994;293:753–758.
- Tits J, Wieland E, Bradbury MH, et al. The uptake of Eu(III) and Th(IV) by calcite under hyperalkaline conditions. Villigen (Switzerland): Paul Scherrer Institut; 2002. ( PSI Report No. 02-03).
- Felmy AR. Chemical speciation of americium, curium and selected tetravalent actinides in high level waste. Richland (WA): PNNL; 2004. ( EMSP Project 73749).
- Tits J, Wieland E, Bradbury MH. The effect of isosaccharinic acid and gluconic acid on the retention of Eu(III), Am(III) and Th(IV) by calcite. Appl Geochem. 2005;20:2082–2096.
- Rojo H, Tits J, Gaona X, et al. Thermodynamics of Np(IV) complexes with gluconic acid under alkaline conditions: sorption studies. Radiochim Acta. 2013;101:133–138.
- Hummel W, Anderegg G, Rao L, et al. Chemical thermodynamics of compounds and complexes of U, Np, Pu, Am, Tc, Se, Ni and Zr with selected organic ligands. In: Mompean FJ, Illemassene M, Perrone J, editors. Chemical thermodynamics. Vol. 9. North-Holland (Amsterdam): Elsevier; 2005.
- Rai D, Rao L, Moore RC, et al. Development of biodegradable isosaccharinate-containing foams for decontamination of actinides: thermodynamic and kinetic reactions between isosaccharinate and actinides on metal and concrete surfaces. Washington (DC): USDOE; 2004. (USDOE Technical Report No. EMSP-82715-2004).
- Warwick P, Evans N, Hall T, et al. Stability constants of uranium (IV)-α-isosaccharinic acid and gluconic acid complexes. Radiochim Acta. 2004;92:897–902.
- Greenfield BF, Holtom GJ, Hurdus MH, et al. The identification and degradation of isosaccharinic acid, a cellulose degradation product. Mat Res Soc Sym. 1995;353:1151–1158.
- Moreton AD, Pilkington NJ, Tweed CJ. Thermodynamic modeling of the effect of hydroxycarboxylic acids on the solubility of plutonium at high pH. Oxfordshire: United Kingdom Nirex Ltd.; 2000. (UK NIREX Report NSS/R339).
- Rai D, Yui M, Moore DA, et al. Thermodynamic model for ThO2(am) solubility in isosaccharinate solutions. J Solution Chem. 2009;38:1573–1587.
- Gaona X, Montoya V, Colas E, et al. Review of the complexation of tetravalent actinides by ISA and gluconate under alkaline to hyperalkaline conditions. J Contam Hydrol. 2008;102:217–227.
- Fanghänel Th, Neck V. Aquatic chemistry and solubility phenomena of actinide oxides/hydroxides. Pure Appl Chem. 2002;74:1895–1907.
- Altmaier M, Gaona X, Fanghänel Th. Recent advances in aqueous actinide chemistry and thermodynamics. Chem Rev. 2013;113:901–943.
- Knope KE, Soderholm L. Solution and solid-state structural chemistry of actinide hydrates and their hydrolysis and condensation products. Chem Rev. 2013;113:944–994.
- Whistler RL, BeMiller JN. α-D-isosaccharino-1,4-lactone. Action of lime water on lactose. In: Whistler RL, Wolfrom ML, BeMiller JN, editors. Methods in carbohydrate chemistry. Vol. II, Reactions of carbohydrates. New York: Academic Press; 1963.
- Ekberg C, Kallvenius G, Albinsson Y, et al. Studies on the hydrolytic behavior of zirconium(IV). J Solution Chem. 2004;33:47–79.
- Sasaki T, Kobayashi T, Takagi I, et al. Solubility measurement of zirconium(IV) hydrous oxide. Radiochim Acta. 2006;94:489–494.
- Altmaier M, Neck V, Fanghänel Th. Solubility of Zr (IV), Th (IV) and Pu (IV) hydrous oxides in CaCl2 solutions and the formation of ternary Ca-M (IV)-OH complexes. Radiochim Acta. 2008;96:541–550.
- Zhang Z, Gibson P, Clark SB, et al. Lactonization and protonation of gluconic acid: a thermodynamic and kinetic study by potentiometry, NMR and ESI-MS. J Solution Chem. 2007;36:1187–1200.
- Colas E, Grive M, Rojo I, et al. Solubility of ThO2 ·xH2O(am) in the presence of gluconate. Radiochim Acta. 2011;99:269–273.
- Kobayashi T, Sasaki T, Takagi I, et al. Solubility and solubility-limiting solid phase in M(IV)-OH-dicarboxylate ternary aqueous system. J Nucl Sci Technol. 2011;48:993–1003.
- Smith RM. NIST critically selected stability constants of metal complexes database version 5.0. Gaithersburg (MD): National Institute of Standards & Technology, U.S. Secretary of Commerce; 1998.
- Sasaki T, Kobayashi T, Takagi I, et al. Hydrolysis constant and coordination geometry of zirconium(IV). J Nucl Sci Technol. 2008;45:735–739.
- Guillaumont R, Fanghänel Th, Fuger J, et al. Update on the chemical thermodynamics of uranium, neptunium, plutonium, americium and technetium. In: Mompean FJ, Illemassene M, Domenech-Orti C, Ben-Said K, editors. Chemical thermodynamics. Vol. 5. Amsterdam: Elsevier; 2003.
- Curti E. Nagra/PSI thermochemical database update: selection of data for zirconium. Villigen (Switzerland): Paul Scherrer Institut; 2001. (Paul Scherrer Institut Report TM-44-01-01).
- Fujiwara K, Yamana H, Fujii T, et al. Determination of uranium(IV) hydrolysis constants and solubility product of UO2*xH2O. Radiochim Acta. 2003;91:345–350.
- Kobayashi T, Sasaki T, Takagi I, et al. Zirconium solubility in ternary aqueous system of Zr(IV)-OH –carboxylates. J Nucl Sci Technol. 2009;46:142–148.
- Rai D, Hess NJ, Xia Y, et al. Comprehensive thermodynamic model applicable to highly acidic to basic conditions for isosaccharinate reactions with Ca(II) and Np(IV). J Solution Chem. 2003;32:665–689.
- Kobayashi T, Sasaki T, Takagi I, et al. Solubility of thorium(IV) in the presence of oxalic and malonic acids. J Sci Nucl Technol. 2009;46:1085–1090.
- Sasaki T, Takaoka Y, Kobayashi T, et al. Hydrolysis constants and complexation of Th(IV) with carboxylates. Radiochim Acta. 2008;96:799–803.
- Vercammen K, Glaus MA, Van Loon LR. Complexation of Th(IV) and Eu(III) by α-isosaccharinic acid under alkaline conditions. Radiochim Acta. 2001;89:393–401.

![Figure 6. Zr solubility at pHc 9.3 and 12.1 after ultrafiltration through 10 kDa membranes as a function of total ISA concentration ([ISA]tot).](/cms/asset/8c2fc40c-1fa3-4ab3-ba79-db85a78b75b3/tnst_a_1255573_f0006_b.gif)