Abstract
Many C3 grasses establish mutualistic associations with endophytic fungi Neotyphodium spp. Lolium multiflorum Lam. natural diploid populations of the Pampa region present high levels of infection whereas commercial cultivars, mainly tetraploid, exhibit low or no infection. In the genus Bromus, no information is available about this association in polyploid populations. The incidence of Neotyphodium spp. endophytes in diploid and tetraploid commercial cultivars of L. multiflorum, as well as in natural hexaploid populations of genus Bromus, was analysed. Observed infection was higher in diploid populations of L. multiflorum than in tetraploid populations. No infection was detected in Bromus spp. Various factors such as the production and handling of commercial seeds, the interaction of endophytes with ploidy level, and the genetic manipulation of seeds are discussed as the possible causes for the differences observed between natural and improved annual ryegrass and brome populations, and between diploid and tetraploid annual ryegrass.
Introduction
Numerous winter grasses establish a mutualistic association with endophytic fungi Neotyphodium Glenn, Bacon and Hanlin (Clavicipitaceae: Ascomycetes) (White Citation1987; Clay & Schardl Citation2002). Such endophytes are asexual and transmitted exclusively vertically through the seeds of host plants. Their life cycle takes place within the host plant, with no symptoms of disease or external reproductive structures (Siegel et al. Citation1987; Roberts et al. Citation2005). Plants infected with these endophytes (E+) exhibit increased tolerance to biotic (Clay Citation1988; Clarke et al. Citation2006; Herre et al. Citation2007; Kuldau & Bacon Citation2008; Popay Citation2009) and abiotic (Clay Citation1993; Malinowski et al. Citation2005; Zabalgogeazcoa Citation2008) stress, associated with the production of different phenolic compounds with antioxidant capacity (Malinowski & Belesky Citation2006; White & Torres Citation2010; Torres et al. Citation2012). In addition, E+ plants exhibit greater adaptation to different geographic environments (Alvarez-Loayza et al. Citation2008; Rodriguez et al. Citation2009) and an increased growth capacity (Schardl et al. Citation2004; Rodriguez et al. Citation2009).
Lolium multiflorum Lam. (Italian, Criollo or annual ryegrass) is a species presenting high forage quality, widely found in the temperate–humid region of Argentina (Beltramino et al. Citation2005). It is originally a diploid species (2n = 2x = 14). However, considering that polyploid species generally have larger organs and that they are larger in size than diploid species, the production of tetraploid cultivars (2n = 4x = 28) is of great interest in order to obtain higher forage yields (Amigone & Tomaso Citation2006; Devo Citation2012).
In Argentina, there has been a growing interest in L. multiflorum, which is reflected in the increasing number of cultivars registered in the National Register of Cultivars from the National Seed Institute (INASE), as well as in higher volumes of trade at a local level. In the period 1983–2012, 112 cultivars were enrolled, of which 57 were tetraploid and 55 diploid (INASE Citation2012).
The endophytic fungi isolated and described in L. multiflorum is N. occultans Moon.
The high incidence of such endophytic fungi in naturalised populations (Colabelli et al. Citation2007) leads to the assumption that endophytic infection allows the plant to adapt to differing environmental conditions. However, commercial cultivars of this species (mainly tetraploid) surprisingly exhibit low or no infection.
Bromus, on the other hand, comprises a large number of species of extremely varied geographical origins (Gillet Citation1983). The basic chromosome number is x = 7, and its species can have different ploidy levels: 2x, 4x, 6x, 8x, 10x and 12x (Gutiérrez & Pensiero Citation1998). In Argentina, Neotyphodium endophytes have been isolated and characterised in Bromus species B. brachyanthera, B. auleticus and B. setifolius (Cabral et al. Citation1999; Iannone et al. Citation2009). However, these endophytes have not been detected in B. catharticus var. rupestris, B. japonicus, B. madritensis and B. pellitus (Iannone et al. Citation2011). The presence of Neotyphodium has been detected in B. catharticus var. catharticus (Colabelli et al. Citation2007), but no characterisation has been made.
From the point of view of the conservation and evaluation of genetic resources, identification of endosymbionts in the seeds of forage grasses is important in order to have germplasm valuable for breeding programmes, mainly in consideration of the adaptation advantage that Neotyphodium endophytes confer to infected plants against biotic and abiotic stress factors.
This study was conducted in order to analyse and compare the incidence of Neotyphodium endophytes in cultivars of diploid and tetraploid annual ryegrass, and in seeds of native populations and commercial cultivars of Bromus spp.
Materials and methods
Cultivars analysed
Seed samples of 55 commercial cultivars of L. multiflorum were analysed, out of which 17 were diploid (2n = 2x = 14) and 38 were tetraploid (2n = 4x = 28). Seed samples of 12 hexaploid populations of Bromus (Barcellini Citation2010) (2n = 6x = 42), known as brome, were also analysed.
Lolium cultivars studied were provided by different seed companies (). As for the 12 populations of Bromus analysed (), eight were native populations of the Buenos Aires province, provided by the Germplasm Bank of INTA Balcarce, southeast of the province of Buenos Aires; two were native populations of the Patagonian region, provided by the Germplasm Bank at INTA Alto Valle; and two were commercial cultivars. The 12 populations of Brome within the section Ceratochloa were Bromus catharticus of different botanical varieties: B. catharticus var. catharticus, B. catharticus var. elata, B. catharticus var. rupestris, B. bonariensis, B. parodii and B. coloratus.
Table 1 Diploid and tetraploid Lolium multiflorum cultivars analysed for the presence of endophytes, and the seed company to which each cultivar belongs.
Table 2 Germplasm accession number, collection site and source of the populations of Bromus spp. analysed.
All seed samples analysed after harvest were kept in a cold chamber at 4 °C and 4%–5% relative humidity to ensure their viability and genetic integrity.
Detection of endophytes by direct staining of tissues
The presence of Neotyphodium spp. was diagnosed in seeds (botanically, caryopses). A sample of 50 seeds per population was randomly taken and analysed. Seeds were pre-treated by soaking in sodium hydroxide at 5%, and they were held at room temperature for 12 h. After this period, they were rinsed with tap water. Each seed was then placed on a microscopic slide. Bracts were separated by means of a pair of histological sterilised needles, and then stained with a drop of Rose Bengal for 2–3 min. Finally, a coverslip was placed over each preparation (Saha et al. Citation1988; Peretti Citation1994).
The methodology allowed the detection of the presence of endophytes in seeds, regardless of the viability of the fungus. Thus, it is possible to diagnose E+ seeds that have been stored for a long period of time, even though the endophyte viability is lost. Taking into account the conditions in which seeds were stored (cold chamber), it is expected that the viability of the fungus is not lost because when the seeds are kept in optimum storage conditions, endophytes can remain viable for many years (Bylin Citation2014, De Battista, pers.comm. 2012).
Microscopic observation
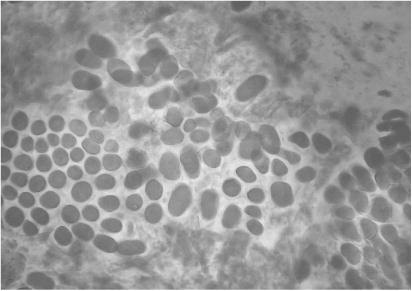
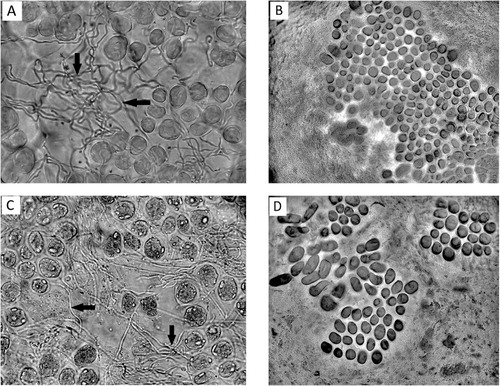
Observations were made under an optical microscope Olympus CHB with magnifications of 10×, 40× and 400×. The presence or absence of stained hyphae in aleurone cells in the peripheral part of the caryopsis was recorded.
Seeds infected with endophyte were diagnosed as positive (E+), and those which were not infected were diagnosed as negative (E–). The percentage of infected seeds per sample was calculated using the following formula:
Statistical analysis
To analyse the effect of ploidy level on infection rates, a contingency table was constructed.
The determination of significant differences between the two groups of L. multiflorum cultivars analysed (diploid and tetraploid) was carried out by performing a Pearson's Chi-squared test. All statistical analyses were carried out using R software (R Development Core Team Citation2011).
Results and discussion
Incidence of Neotyphodium spp.
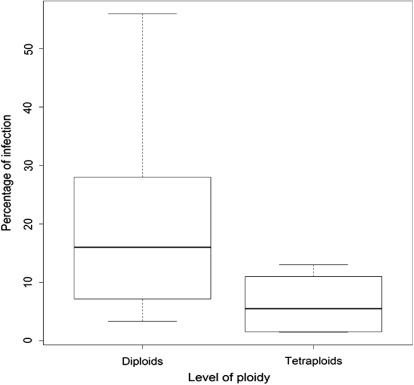
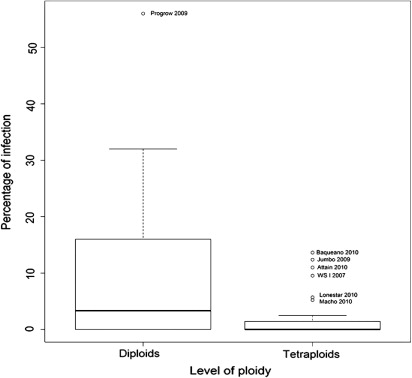
For the species L. multiflorum, endophyte hyphae were detected both in the diploid and tetraploid cultivars tested (; –). However, endophyte incidence was greater for diploid than for tetraploid cultivars analysed. Infection was detected in 52.9% and 26.3% of diploid and tetraploid cultivars, respectively (P < 0.1). On the other hand, average infection in diploid cultivars was 10.8%, ranging from 0%–56%, whereas average infection in tetraploid cultivars was 1.8%, ranging from 0%–13% (P < 0.001, ). When the endophytic infection rate was compared for only those cultivars that showed infection, the average infection for diploid cultivars was 16.92%, whereas for tetraploid cultivars the infection rate was 5.8% (P < 0.001, ). With respect to Bromus, no Neotyphodium hyphae were detected in accessions from the Forage Germplasm Banks of INTA or in the commercial cultivars evaluated (Gato and Volcan) (; ).
Table 3 Infection rate (%) in seed samples of diploid cultivars of Lolium multiflorum Lam.
Table 4 Infection rate (%) in seed samples of tetraploid cultivars of Lolium multiflorum Lam.
Table 5 Infection rate (%) in seed samples of hexaploid populations of Bromus.
Effect of ploidy level
When the endophytic infection rate was compared for all cultivars tested, average infection was 9 percentage points higher for diploid than for tetraploid cultivars. This difference between the two groups of cultivars analysed was significant (P < 0.001). Similarly, when the endophytic infection rate was compared for only those cultivars that showed infection, average infection was 11.12 percentage points higher for diploid than for tetraploid cultivars. This difference between the two groups of cultivars analysed was also significant (P < 0.001). This shows that the endophytic infection rate was significantly higher in diploid populations than in the commercial tetraploid populations studied.
The results presented in this work coincide with those of De Battista (pers. comm. 2012). This suggests that the lower ploidy level in annual ryegrass may result in a greater net benefit from the relationship with the endophyte.
Whereas Bromus polyploidy is a natural event, in Lolium no natural polyploid exists; it is artificially achieved using colchicine as inductor. Induction of chromosome doubling is of interest to forage breeding programmes as it is used to meet different purposes, such as nutritional value, forage production, distribution of forage production depending on the vegetative cycle, resistance to pests and diseases, tolerance to abiotic stress and fertility restoration of sterile hybrids (Nair Citation2004; Campos et al. Citation2009; Pereira et al. Citation2012). Humphreys (Citation1991) found that chromosome doubling directly affects the performance of plants through increases in the size of cells and vacuoles. This usually gives tetraploid cultivars greater hardiness, drought and pest tolerance than diploid cultivars. The seed size is also increased, leading to greater seedling vigour and establishment potential. It is unknown whether the application of colchicine may have a toxic effect on the development of the fungus. However, if colchicine phytotoxicity exists, it cannot be as pronounced, because infected tetraploid cultivars were detected.
For diploid cultivars, results differ sharply from those found in naturalised populations, where almost 100% presented high levels (>50%) of endophyte infection (Colabelli et al. Citation2007; Gundel et al. Citation2008). One might speculate that the anthropic selection favoured characteristics different from those favoured by natural selection, and so it did not favour or eliminate infection in improved populations. Furthermore, taking into account the difference in viability of the seeds and the fungus in the plant, the fungus might have been lost as a result of manipulations in the seed pools of breeding programmes, so that no reinfection occurred.
Overall, tetraploid cultivars had a low infection rate. It is noteworthy that samples from different years of the same cultivar (Baqueano, Jumbo) showed both maximum values (12%–13%) and values of 0% infection. It is well known that dissemination of these fungal symbionts occurs only through the seeds, so it is possible that positive seeds found in some tetraploid samples were contaminated with diploid naturalised ryegrass in the breeding farms. On the other hand, morpho-physiological changes produced by autopolyploidy could reduce the competitive advantage given by the endosymbiont and, in such cases, the net balance of the symbiosis would be negative in terms of energy and nutritional balance of the host.
As regards the genus Bromus, the nil effect of endosymbionts both in native populations and commercial cultivars assessed shows a lack of variability in the association with Neotyphodium within and among the species studied. The percentage of zero infection found in this work for B. catharticus var. rupestris (= B. brevis Nees ex Steud) coincides with that recorded by Colabelli et al. (Citation2007) for native populations of this species from the humid Pampa. However, in populations of B. catharticus var. catharticus, the zero rate of infection does not coincide with that obtained by Piontelli & Toro (Citation1992) and Colabelli et al. (Citation2007) who detected endophyte infection. It is important to mention that in the analyses by Colabelli et al. (Citation2007) in germplasm collections, the frequency of infection of B. catharticus var. catharticus was very low: 6% (1 in 17 cultivars analysed), and the population studied in this work showed a relatively low level of infection (15%).
Although what we propose in this study requires further evaluation, partial results indicate that a higher level of ploidy on hosts decreases the incidence of infection with Neotyphodium endophytes. Further studies are needed to better understand the distribution and incidence of the fungus in this genus and to determine whether they can be directly associated to the ploidy level of the host.
Conclusion
A higher incidence of infection with Neotyphodium endophytes was found in diploid commercial populations of L. multiflorum than in tetraploid cultivars. No inter- or intra-specific variability was observed for Bromus in association with Neotyphodium endophytes.
Acknowledgements
To the memory of the Agr. Eng. José P. De Battista. We thank Dr Silvina San Martino for collaborating in the statistical analysis.
References
- Alvarez-Loayza P, White Jr JF, Gil N, Svenning JC, Balslev H, Kristiansen T 2008. Light converts endosymbiotic fungus to pathogen, influencing seedling survival and host tree recruitment 2. http://precedings.nature.com/documents/1908/version/1 (accessed 20 December 2013).
- Amigone MA, Tomaso JC 2006. Principales características de especies y cultivares de verdeos invernales. Informe para Extensión. http://inta.gob.ar/documentos/principales-caracteristicas-de-especies-y-cultivares-de-verdeos-invernales/ (accessed 16 December 2013).
- Andrés AN, De Battista JJ, Ivancovich AJ 2000. Mejoramiento y evaluación de raigrás anual. Reunión Anual de Forrajeras, Pergamino, Argentina, 30 de octubre de 2000. Pp. 16–24.
- Barcellini L 2010. Evaluación de poblaciones de Bromus spp. (Secc. Ceratochloa). II—Nivel de ploidía, fenología y persistencia. Tesis inédita de grado, Balcarce, Argentina, Facultad de Ciencias Agrarias, Universidad Nacional de Mar del Plata. 33 p.
- Beltramino HJ, Medvescigh JC, De Battista JJ, Costa M 2005. Efecto del hongo endófito Neotyphodium occultans en la producción de semilla de raigrás anual. RCA. Revista Científica Agropecuaria 9: 25–31.
- Bylin A 2014. Endophytic fungi in meadow fescue and other forage grasses. Doctoral thesis. Umeå, Faculty of Natural Resources and Agricultural Sciences, Department of Agricultural Research for Northern Sweden. 56 p.
- Cabral D, Cafaro MJ, Saidman B, Lugo M, Reddy PV, White Jr JF 1999. Evidence supporting the occurrence of a new species of endophyte in some South American grasses. Mycologia 91: 315–325.10.2307/3761376
- Campos JMS, Davide LC, Salgado CC, Santos FC, Costa PN, Silva PS et al. 2009. In vitro induction of hexaploid plants from triploid hybrids of Pennisetum purpureum and Pennisetum glaucum. Plant Breeding 128: 101–104.10.1111/j.1439-0523.2008.01546.x
- Clarke BB, White Jr JF, Hurley RH, Torres MS, Sun S, Huff DR 2006. Endophyte-mediated suppression of dollar spot disease in fine fescues. Plant Disease 90: 994–998.10.1094/PD-90-0994
- Clay K 1988. Fungal endophytes of grasses: a defensive mutualism between plants and fungi. Ecology 69: 10–16.10.2307/1943155
- Clay K 1993. The ecology and evolution of endophytes. Agriculture, Ecosystems & Environment 44: 39–64.10.1016/0167-8809(93)90038-Q
- Clay K, Schardl C 2002. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. The American Naturalist 160: 99–127.10.1086/342161
- Colabelli MN, Clausen AM, De Battista J, Costa M, Torres MS, Re A et al. 2007. Incidencia de endófitos tipo Neotyphodium en forrajeras nativas y naturalizadas e impacto agronómico en Lolium multiflorum Lam. en la Argentina. Avances de Investigación de Recursos Genéticos en el Cono Sur II. IICA-PROCISUR, Montevideo, Uruguay. Pp. 45–55.
- Devo MA 2012. Efecto de la duplicación cromosómica sobre caracteres vegetativos y reproductivos de una población naturalizada de Lolium multiflorum Lam. Tesis inédita de grado. Balcarce, Argentina, Facultad de Ciencias Agrarias, Universidad Nacional de Mar del Plata. 32 p.
- Gillet M 1983. Las gramíneas forrajeras: descripción, funcionamiento, aplicaciones al cultivo de la hierba. Zaragoza, España, Acribia. 354 p.
- Gundel PE, Batista WB, Texeira M, Martinez-Ghersa MA, Omacini M, Ghersa CM 2008. Neotyphodium endophyte infection frequency in annual grass populations: relative importance of mutualism and transmission efficiency. Proceedings of the Royal Society B: Biological Sciences 275: 897–905.10.1111/j.1469-8137.2007.01978.x
- Gutiérrez HF, Pensiero JF 1998. Sinopsis de las especies argentinas del género Bromus (Poaceae). Darwiniana, nueva serie 35: 75–114.
- Herre EA, Mejía LC, Kyllo DA, Rojas E, Maynard Z, Butler A 2007. Ecological implications of anti-pathogen effects of tropical fungal endophytes and mycorrhizae. Ecology 88: 550–558.10.1890/05-1606
- Humphreys M 1991. Genetic control of physiological response—a necessary relationship. Functional Ecology 5: 213–221.
- Iannone LJ, Cabral D, Schardl CL, Rossi MS 2009. Phylogenetic divergence, morphological and physiological differences distinguish a new Neotyphodium endophyte species in the grass Bromus auleticus from South America. Mycologia 101: 340–351. http://www.mycologia.org/content/101/3/340.full.pdf+html ( accessed 26 November 2013).
- Iannone LJ, White Jr JF, Giussani LM, Cabral D, Novas MV 2011. Diversity and distribution of Neotyphodium-infected grasses in Argentina. Mycological Progress 10: 9–19.10.1007/s11557-010-0669-2
- Instituto Nacional de Semillas (INASE) 2012. Registro de Variedades. Catálogo Nacional de Cultivares 2012. http://www.inase.gov.ar/consultaGestion/gestiones ( accessed 15 December 2012).
- Kuldau G, Bacon C 2008. Clavicipitaceous endophytes: their ability to enhance resistance of grasses to multiple stresses. Biological Control 46: 57–71.10.1016/j.biocontrol.2008.01.023
- Malinowski DP, Belesky DP 2006. Ecological importance of Neotyphodium spp. grass endophytes in agroecosystems. Grassland Science 52: 1–14.10.1111/j.1744-697X.2006.00041.x
- Malinowski MP, Belesky DP, Lewis GC 2005. Abiotic Neotyphodium stresses in endophytic grasses. In: Roberts C, West CP, Spiers DE eds. Neotyphodium in cool-season grasses. Ames, OH, Blackwell Publishing. Pp. 187–199.
- Nair RM 2004. Developing tetraploid perennial ryegrass (Lolium perenne L.) populations. New Zealand Journal of Agricultural Research 47: 45–49.10.1080/00288233.2004.9513569
- Pereira RC, Davide LC, Techio VH, Timbó ALO 2012. Duplicação cromossômica de gramíneas forrageiras: uma alternativa para programas de melhoramento genético. Ciência Rural 42: 1278–1285. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-84782012000700023&lng=en&tlng=pt.%2010.1590/S0103-84782012000700023 ( accessed 28 November 2013).
- Peretti A 1994. Manual para análisis de semillas. Buenos Aires, Argentina, Hemisferio Sur. 281 p.
- Piontelli E, Toro M 1992. Endófitos fúngicos en comunidades de pastos naturales: distribución en algunas especies de Lolium, Festuca, Bromus y Stipa. Revista Simiente 63: 127.
- Popay AJ 2009. Insect herbivory and defensive mutualism between plants and fungi. In: White Jr JF, Torres MS eds. Defensive mutualism in microbial symbiosis. Boca Raton, FL, CRC Press. Pp. 347–358.
- R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing. http://www.R-project.org/ (accessed 3 November 2013).
- Roberts CA, West CP, Spiers DE 2005. Neotyphodium in cool-season grasses. Oxford, Blackwell.
- Rodriguez R, White Jr JF, Arnold AE, Redman RS 2009. Fungal endophytes: diversity and functional roles. New Phytologist 182: 314–330.10.1111/j.1469-8137.2009.02773.x
- Saha CD, Jackson MA, Johnson Cicalese JM 1988. A rapid staining method for detection of endophytic fungi in turf and forage grass. Phytopathology 78: 237–239.
- Schardl CL, Leuchtmann A, Spiering MJ 2004. Symbioses of grasses with seedborne fungal endophytes. Annual Review of Plant Biology 55: 315–340.10.1146/annurev.arplant.55.031903.141735
- Siegel MR, Latch GCM, Johnson MC 1987. Fungal endophytes of grasses. Annual Review of Phytopathology 25: 293–315.10.1146/annurev.py.25.090187.001453
- Torres MS, White JF Jr, Zhang X, Hinton DM, Bacon CW 2012. Endophyte-mediated adjustments in host morphology and physiology and effects on host fitness traits in grasses. Fungal Ecology 5: 322–330.10.1016/j.funeco.2011.05.006
- White JF Jr 1987. Whe widespread distribution of endophytes in Poaceae. Plant Disease 71: 340–342.10.1094/PD-71-0340
- White JF Jr, Torres MS 2010. Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiologia Plantarum 138: 440–446.
- Zabalgogeazcoa I 2008. Fungal endophytes and their interaction with plant pathogens. Spanish Journal of Agricultural Research 6: 138–146.