ABSTRACT
The microbiology within soils supports a wide range of ecosystems underpinning the productive capacity and environmental sustainability of land use. However, we have yet to make significant gains in the management of soil biology to achieve multiple gains across ecosystem services. Recent advances in environmental metagenomics are now enabling the characterisation of complex soil ecosystems. Advances in tools to characterise ecosystems by their DNA or RNA, alongside new approaches for the analysis of this information, are being integrated with environmental biogeochemistry to provide new insights into the functional assessment of microbial communities in relation to soil function and ecosystem services. Using these approaches, real-time assessment of drivers affecting soil phenotype (i.e. collective expression of multiple functions) can be assessed in relation to management and environmental changes, and opportunities for controlled alteration of soils for productive and environmental gains may be achieved.
Introduction
To state that soils are ‘highly diverse’ grossly understates the richness of species that inhabit this ecosystem. In fact, it is probable that soils are the most species-rich habitats on Earth (Curtis et al. Citation2002; Torsvik et al. Citation2002). However, as most of this life is at the microbial scale we rarely directly see it (). This is unfortunate given the fundamental role the microbiology in soil has for sustaining processes spanning global biogeochemical cycling through to the productivity of land uses associated with primary production (Bardgett and van der Putten Citation2014), and even in affecting human health (Brevik and Sauer Citation2015; Steffan et al. Citation2017). Furthermore, our lack of ability to directly visualise soil microbiology means that soil microbiology, and the functions and ecosystems it supports, is conceptually abstract to most people, very much the proverbial ‘black box’.
Figure 1. Much of life in soil exists at the micrometer scale, although fruiting bodies of fungi, and colonies (swarms) of bacteria, and are sometimes visible to the naked eye. Given our inability to directly observe soil biology, we cannot appreciate the diversity present, or directly observe the links between changes in management practices and soil ecosystems. Our ability to observe phenotypes of animals and plants has enabled selection of enhanced breeds and cultivars over time. As such, in order to make similar gains in soil microbial resources, approaches such as those based on environmental genomics approaches will be needed.
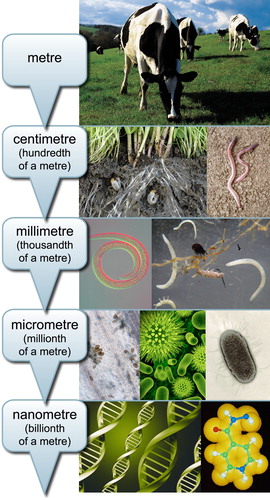
However, the development of new analytical tools, particularly those based on environmental DNA/RNA analysis, metabolomics and measurement of soil function using integrative methods such as stable-isotope probing (van Straalen and Roelofs Citation2006), are revolutionising our ability to manage soils for directed outcomes. These will provide new opportunities to increase sustainability and profitability of the agricultural, horticultural and forestry sectors (Wakelin, Cave, et al. Citation2016). This paper reviews the extent of diversity of soil microbiology in soils, how diversity links with functional processes, ecosystem services and resilience. Finally, the role of ecological genomics and integrative approaches to characterise soils across multiple phenotypes are discussed, and opportunities to manage soil to optimise productivity and sustainably presented therein.
Diversity of microbiology in soils
Soil ecosystems comprise complex communities of bacteria, archaea and eukaryotic taxa. Bacteria and archaea are typically microscopic () and are generally not visible to the naked eye. Even the domain Eukaryia, the phylogenetic home to higher animals and plants, is dominated by microbial taxa, many of which have species that can be found in soil. These include small animals such as nematodes, collembolan, isopods, through to algae, cercozoa (e.g. some protozoa), and, of course, fungi. The diversity of microbial life in soil, across these three domains, is incredibly high. Although estimates of total richness vary, for the Bacteria domain alone a general consensus is that thousands to tens-of-thousands of species are present in each gram of agricultural soil (Curtis et al. Citation2002; Torsvik et al. Citation2002; Schloss and Handelsman Citation2006). Incredibly, in a handful of soil, a farmer can be holding more diversity of life that, for example, the plants and animals present in a rainforest (Dirzo and Raven Citation2003). The overlap of microbial species between soils can be surprisingly low. A comparison between soil from Minnesota and Alaska, for example, found only 20% of shared species; most were endemic to the site from which they were sampled (Schloss and Handelsman Citation2006). Furthermore, many of the microbial species present in soil are at low abundance, and species-abundance curves typically have a very long tail: The deeper we look, the more we discover.
Over the last decade, there has been considerable research interest in determining the factors associated with the high total diversity of microbial life in soil distribution in community assemblage among soils. Many studies have demonstrated the importance of many individual factors, such as tillage, land-use change, agrichemical input, soil mineralogy, crop-residue management, stocking rate, land-use history, crop-based selection, plant growth stage, seasonality and so forth (e.g. Robertson et al. Citation1997; Marschner et al. Citation2001; Garbeva et al. Citation2004; Bunemann et al. Citation2006; Wakelin et al. Citation2007; Eilers et al. Citation2010; Jangid et al. Citation2011; Hannula et la. Citation2012; Reith et al. Citation2012). However, many of these studies have been constrained with respect to geographic range and/or environmental properties under investigation. Thus, when constrained to a relatively narrow geographical range, factors such as above-ground botanical composition can constitute a significant driver (e.g. Smalla et al. Citation2001). Similarly, when botanical composition is constrained, grazing intensity or fertiliser inputs can be shown to be important (e.g. Wakelin et al. Citation2009). As such, nearly all conceivable factors tested to date have been shown to have a measurable impact on the composition of microbial species in soils. It has only been through survey-based sampling approaches (e.g. Lauber et al. Citation2008; Wakelin et al. Citation2008) that the relative contribution of a range of potential drivers can be assessed (e.g. soil, plant, environment), and links back to primary variables (e.g. soil pH) be determined. Indeed, there is now increasing evidence that pH is one of the principal factors controlling the range and richness of species present in soils (Lauber et al. Citation2008; Wakelin et al. Citation2008; Kuramae et al. Citation2012). Following from the influence of soil pH are other factors such as nitrogen and carbon availability, temperature and REDOX (reviewed in Fierer Citation2017). From within this pool of diversity, plants may selectively enrich microbiota on and adjacent to their root systems based on metabolic cues released to the soil (Bulgarelli et al. Citation2012). In general, the bulk soil conditions largely establish the species comprising the soil meta-community, and from within this, plants select a rhizosphere community, then rhizoplane community and so on. However, these and other effects are reviewed in more detail below.
Variation in soil bacteria community composition
A number of soil properties have been associated with bacterial richness and composition; for soil bacteria, as mentioned before, pH appears to be a master variable. Soil pH was highly correlated with similarity in the composition of bacterial communities in agricultural soil samples across three Australian states (Wakelin et al. Citation2008). In the same samples, patterns in soil catabolic function (mineralisation of a range of carbon sources) were also strongly associated with soil pH, and these functional changes were associated back to changes in bacterial community structure through soil pH. When investigating the influence of land-use change on soil microbial communities, Lauber et al. (Citation2008) and Kuramae et al. (Citation2012) independently established that observed ‘land-use’ effects on the bacteria present were driven through alteration in soil pH. This is supported by work sampled across very broad geographical distances (continental to trans-continental surveys) demonstrating the primary importance of soil pH, and secondary to this the importance of factors such as distance (spatial), temperature and so forth (Fierer and Jackson Citation2006; Lauber et al. Citation2009). Bissett et al. (Citation2010) collected ∼60 samples of soil from across New South Wales, Australia. The samples were taken from over a wide geographical range but were deliberately highly constrained with regard to edaphic properties, particularly soil pH. When looking across the ‘total’ bacterial community, spatial variation (distance between samples) emerged as an important factor – i.e. communities were more dissimilar with distance – and the major factors associated with distance were climatic (rainfall and temperature) and environmental (moisture), and then nutritional. Given agricultural soils are (generally) tightly managed to a narrow pH range, the importance of environmental factors on driving bacterial diversity may be relatively greater than results indicated by surveys covering assortments of different land uses.
Not surprisingly, at different taxonomic levels, the impacts of a range of other soil and environmental properties are evident. The abundance of several bacterial taxa has been linked to the carbon content and quality of soils (Fierer et al. Citation2007; Eilers et al. Citation2010), indicating the presence of K- and r-type life strategies in these organisms. The response of bacterial taxa to edaphic or environmental properties can also vary greatly depending on the bacterial taxa involved (Bissett et al. Citation2010), such that consideration on the relative importance of factors such as ‘soil type’ vs. ‘climate’ both depend on the taxonomic resolution (e.g. genera of associative N-fixing Rhizobia spp. c.f. total Proteobacteria) and the life history (poor v good dispersers). This is particularly important for considering potential impacts of management practices on soil biology in agroecosystems, where the soil ecosystem services may be supported by either very narrow or broad range of taxa.
Variation in soil fungal community composition
Unlike bacteria, species of soil fungi generally have a wide tolerance of pH range (e.g. Wheeler et al. Citation1991). As such, soil pH often has a lower total associative correlation with fungal community composition than associations for bacteria in the same soil samples (Wakelin et al. Citation2008; Rousk et al. Citation2010). Despite this, soil pH still remains one of the most important properties linked with fungal community composition in agricultural (Wakelin et al. Citation2008, Citation2009; Rousk et al. Citation2010) and grassland soils (Wakelin, Barratt, et al. Citation2013). The lack of ‘other’ strongly associative factors in these surveys indicates that either properties not measured, and therefore not included in the analyses, were important (e.g. plant residue resource quality), or the occurrence of strong stochastic elements in the assemblage of these communities.
In agricultural soils, the total fungal community composition is often more strongly responsive to management practices than the bacterial community from the same soils (Wakelin et al. Citation2009), particularly when inputs of plant residues are assessed (Wakelin et al. Citation2007). Other factors associated with fungal community composition include plant-based influences (Millard and Singh Citation2010), and these effects are particularly strong for the plant-associated arbuscular mycorrhizal fungi (AMF) (Jansa et al. Citation2002). Surveys across agricultural soils have shown that the community structure in soil fungal communities is linked to soil structure (Wakelin et al. Citation2008). Given the invasive growth habit of fungi, soil pore sizes are likely to be important in affecting which species (narrow vs. wide mycelium) can colonise and establish (Harris et al. Citation2003). Similarly, the distribution of plant pathogenic fungi will be influenced by the presence of a susceptible host plant. However, the level of influence is in proportion to the degree of specialisation towards pathogenicity by the fungus, as many also have a degree of saprophytic ability enabling them to grow in the absence of a susceptible host (Garrett Citation1963).
Communities of fungi in agricultural soils exhibit a strong influence by ‘seasonality’, with both species richness and community structure showing strong temporal links (Wakelin et al. Citation2009) which were associated with rainfall variation. Furthermore, in a structured sampling study, the influence of ‘time’ was ranked as the second most important on soil microbial communities (soil type > time > management; Bossio et al. Citation1998). The influence of a time-dependent factor on fungal community composition indicates strong environmental-based influence (temperature, moisture, resource availability) for this group of soil microbiota. Soil fungi also show strong thermal acclimation, whereby certain lineages have varying tolerance ranges of temperature which control respiration and heterotrophic activity/decomposition (Crowther and Bradford, Citation2013).
Alteration in the fungal community is likely to impact on several important ecosystem services. Fungi are the major group associated with decomposition of plant residues and have a greater overall contribution to soil-nutrient cycling than bacteria in most undisturbed soils (Garrett, Citation1963), such as grassland ecosystems (Wakelin, Barratt, et al. Citation2013). In addition to nutrient supply, it has recently been shown that soil fungi are a major source of nitrous oxide (N2O) emissions via denitrification and co-denitrification (Shoun et al. Citation1992; Laughlin and Stevens Citation2002). How widespread this capacity is across the fungal domain is, as yet, unknown; however, alteration in fungal community structure may positively or negatively impact on this process. Better characterised are the roles of soil fungi in plant health (disease and disease suppression; Garbeva et al. Citation2004), soil P solubilisation and mineralisation (Kucey Citation1983; Whitelaw Citation2000), and AMF associations with plant roots which effectively increase the plants’ absorptive surface in contact with soil and aid in P uptake (Jackobsen et al. Citation1992; Marschner and Dell Citation1994). The hyphal networks and complex polysaccharides produced by filamentous fungi are important for formation and stability of soil aggregates (Beare et al. Citation1997) which, in turn, increase soil C storage and soil stability. Structural changes in the saprophytic component of the microbial community are considered central to the soil ecosystem response to environmental change (Zak et al. Citation2011), particularly through alteration of soil organic matter and dissolved organic carbon.
Overall, soil fungi have a vital role underpinning many soil ecosystem services. However, knowledge on the ecology of soil fungi is lacking compared with bacteria, mostly due to slower development of DNA-based tools, and particularly those targeting fungal-driven processes (e.g. fungal N2O emissions).
Other taxa
In addition to bacteria and fungi, the soil microbial community comprises a wide range of other taxa including unicellular Eukaryia such as flagellates, amoebae, algae and so on. These have a vital role in food webs and nutrient cycling. However, knowledge about the ecology of these taxa is scant compared with bacteria and fungi. One of the few studies on global diversity of soil protists was conducted by Bates et al. (Citation2012). The findings established the ubiquitous presence of protists in soils, with levels of diversity reaching that of the bacterial community. Unlike bacterial communities, the assemblage of protistan communities was not related to soil physicochemical properties, but rather climatic conditions associated with soil moisture availability. Thus, these taxa may be particularly susceptible to changes in climate-associated environmental conditions, or alteration of soil moisture with factors such as cover crops, residue retention and irrigation frequency.
Until relatively recently, Archaea were only known to inhabit extreme environments such as salt lakes (halophiles), hot springs (thermophiles) and in acidic mine-drainage (acidophiles). However, using molecular-based phylogenetic methods, it became apparent that archaea are a cosmopolitan component of soil microbial communities (Ueda et al. Citation1995; Bintrim et al. Citation1997; Buckley et al. Citation1998). Although their abundances (Archaeal 16S rRNA gene counts) suggest they comprise only ∼2% of the agricultural soil microbial community (Bates et al. Citation2011), their activities underpin key biogeochemical transformations such as those associated with methanogenesis (Nicol et al. Citation2003; Angel et al. Citation2012) and ammonia oxidation (Leininger et al. Citation2006; Bowatte et al. Citation2009).
While present in all soils, Archaea are notoriously difficult to culture and, as such, knowledge of their ecology is almost entirely based on application of molecular tools, phospholipid markers and other non-cultivable methods (Fierer Citation2017). Only a few studies have investigated factors influencing the diversity and structure of archaea in different agricultural soils. Over a range of agricultural soils under different management, Cao et al. (Citation2012) identified soil pH as a key driver of abundance, possibly through regulation of nutrient availability, the premise based on archaea being adapted to low nutrient/energy habitats (Valentine Citation2007). However, factors controlling community structure were less evident and were likely to be confounded by spatial and climatic factors. In a study of 146 global soil samples, Bates et al. (Citation2011) reaffirmed that archaea are common in agricultural soils and also found that two ‘crenarchaeotal’ phylotypes represented >70% of all archaea present. The abundance of archaea increases with widening C:N ratios in soil; this is consistent with the ecology of some Crenarchaeota taxa as ammonia oxidisers (Leininger et al. Citation2006). The dominance of Crenarchaeota in the global soil samples supports the work of Nicol et al. (Citation2003) who found that Euryarchaeota lineages, associated with methanogenesis, are generally only detectable after anoxic conditions are established. More recently, Angel et al. (Citation2010) investigated the distribution of archaea over a precipitation gradient and the findings demonstrate the importance of climatic region in affecting community composition.
In summary, archaea in agricultural soils are dominated by a few phenotypes, yet these have an important role in soil N and C biogeochemistry. The abundance of these taxa appear to be related to factors associated with energy status of the ecosystem, such as C:N ratio and pH, both of which influence soil ammonia concentrations. The low phylogenetic diversity underpinning this function may mean that archaeal communities, and the ammonia oxidation as their key ‘function’, are particularly susceptible to ecosystem disruption or change.
Ecosystem functions
There is often little value given to the life in soil from a conservation perspective, or in understanding or maintaining the diversity of life present for its intrinsic importance. Indeed, Giller (Citation1996) characterised diversity of life in soils as a ‘poor man’s rainforest’. However, when we consider the range of essential functions that soil microbiology supports, nothing could be further from the truth (Giller Citation1996; Bardgett and van der Puttin Citation2014). Many links between microbial diversity and soil functions have been highlighted in the preceding sections. These functions build to support a wide range of essential ecosystem services that affect the productivity of soils, but also the health of connected ecosystems, such as rivers, groundwater systems and the atmosphere (). Perhaps the best recognised is the role of microorganisms in affecting the cycling of most major and minor elements. In Citation1977, David Jenkinson, publishing in New Zealand Soil News, likened the soil microbial biomass to the ‘eye of the needle’ through which carbon and nutrient transformations are mediated. This phrase is widely used to highlight the role of soil microbiology in the cycling of organic matter, but also nutrient availability and energy flow more widely. Indeed, decomposition is an essential process that drives many ecosystem functions such as carbon cycling and storage, soil formation and stabilisation, disease suppression, regulation of water supply and so forth.
Figure 2. Conceptual diagram of how soil microbial communities and the diversity of species and community composition therein support soil functions and thereby soil ecosystems services.
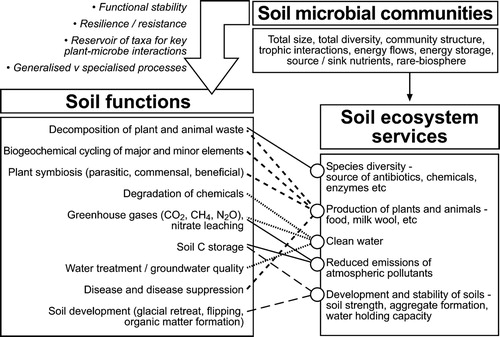
One approach to assess the myriad of functions supported by soil microbiology, and how these are supported by microbial biodiversity, is through an ecosystem services framework approach (). Within this, different functions can be linked to ecosystem outcomes, or services, and these can be grouped. An interesting outcome from using this framework is an appreciation of the high degree of connectivity among a range of functions and individual ecosystem services (), i.e. many ecosystem services are supported by multiple functions.
Complex relationships among diversity and functions
As ecosystem services are underpinned by one or more soil functions, soil functions are supported by a narrow to wide range of microorganisms. For example, the ability to decompose cellulose is widespread among taxonomically diverse lineages of soil microbiota (i.e. a polyphyletic function) (Štursová et al. Citation2012). This is not surprising, as it is the most abundant polymer on earth (Klemm et al. Citation2005) and therefore a major resource for many heterotrophic species. Given the massive degree in redundancy species that have the functional capacity to utilise cellulose, and the polyphyletic nature of this diversity, the disruption of soil ecosystems and even complete loss of some niches would have little enduring impact on cellulose decomposition; it is a relatively resistant and stable ecosystem function.
In contrast, some specific nitrogen transformations are supported by a relatively low taxonomic range of microbial species (potentially monophyletic) that can be of low abundance. A widely recognised example is nitrification, a key biochemical transformation that links supply of nitrogen from mineralisation of soil organic matter (as ammonia) into nitrate-N (Focht and Verstraete Citation1977). The importance of nitrate-N in both plant productivity and potential environmental impacts is well known. A key transformation in the nitrification pathway is ammonia oxidation which, in New Zealand’s pastures, mostly occurs via the activity of a narrow group of chemolithoautotrophic bacteria (Di et al. Citation2009). Given the essential importance of ammonia oxidation to nitrification, and the relatively narrow range of microbial species that can oxidise ammonia, this ecosystem function is particularly susceptible to environmental disturbance (Kowalchuk and Stephen Citation2001). Indeed, ammonia oxidation is widely used as an indicator of ecosystem health and stability (Colloff et al. Citation2008). With increasing disturbance of soils, for example, following addition of heavy metals such as copper or zinc, ammonia-oxidising bacteria are affected and N-cycling disrupted. Recovery of function is typically dependent on the presence of rare taxa (Mertens et al. Citation2009). In systems in which this redundancy in functional diversity is not present, or when conditions do no support the growth of these taxa, ecosystem function is impacted. This can be tested under controlled experimental systems whereby manipulated dilution of diversity reduces terrestrial ecosystem processes, such as potential denitrification rate (Philippot et al. Citation2013). Similarly, the role of the rare soil microbiome in other processes, such as biogeochemical cycling of S, is also recognised (Pester et al. Citation2010).
The examples above show that relationships between microbial diversity and function vary widely (Azarbad et al. Citation2016). The effect of loss of diversity on function ranges from no measurable effect, a linear or non-linear decrease in function, no change until a break-point is reached, are idiosyncratic, or otherwise varied. Importantly, however, most functions do not occur in isolation. For example, decomposition of plant residues (cellulose), a process supported by a plethora of microbial species and therefore having high functional redundancy, can be considered very stable. Yet, to support decomposition of carbon-rich substrates, sufficient N, P and other essential elements must be available. As such, the biogeochemical cycling of a broad range of elements may affect the activity of cellulolytic microorganisms and thereby C-cycling more widely (Henriksen and Breland Citation1999). Given close coupling between these nutrient cycles, the most sensitive point for C-cycling, in terms of loss of function with biodiversity impact and provision of ecosystem services, could be through ammonia oxidation in the nitrogen cycle. It therefore follows that the high degree of sensitivity of ammonia oxidation to ecosystem disturbance confers susceptibility to function in cellulose decomposition. Similar outcomes emerge between interactions of phosphorus (P) and carbon. In soils with low P-availability (i.e. content), an enrichment in microorganisms able to release P bound in the mineral phase can occur (Mander et al. Citation2012). As P-availability increases, constraints in C-cycling were overcome allowing for shifts in ecosystem function following disturbance (Wakelin, MacDonald, et al. Citation2013).
Maintaining a high degree of diversity of microbial life in soils therefore underpins ecosystem function and soil services (Coleman and Whitman Citation2005). Although many ecosystem models treat soil microbiology as a single variable, it is increasingly evident that microbial community composition plays an important role in determining the rates of important ecosystem processes such as trace gas formation (Schimel and Gulledge Citation1998), carbon cycling and sequestration (Waldrop et al. Citation2000; Schmidt et al. Citation2011; Schimel and Schaeffer Citation2012), decomposition (Hendrix et al. Citation1986), N biogeochemistry (Cavigelli and Robertson Citation2000; Balser and Firestone Citation2005) and disease suppression (Mendes et al. Citation2011). The assumption that microbial communities from the same environment behave in a functionally equivalent manner is incorrect (Strickland et al. Citation2009); ecosystem models need to consider both community composition and functional traits to accurately predict outcomes associated with soil function and ecosystem services.
Immediate opportunities for managing soil biological resources for New Zealand
Myriad opportunities exist whereby management of soil biological resources can have important outcomes for New Zealand’s productive sectors. These may be related to increased production, profitability, ecosystem stability or provision of other ecosystem services (see previous). Many of these have been identified as part of previous reviews or other published materials (e.g. Andrews et al. Citation2011; Wakelin, van Koten, et al. Citation2013; Wakelin, Cave, et al., Citation2016). As such, the goal here is not to restate previous considerations, but rather present examples of recent research or new opportunities for soil microbiology-based research across different land-use types in New Zealand, particularly those underpinned by multidisciplinary approaches.
Sheep and beef farming: improving soil rhizobia resources
In New Zealand, expansive pastoral agriculture typically supports grazing of livestock for meat and/or fibre production. Relative to dairy farming, these agricultural systems generally have lower inputs of chemical fertilisers and operate on a lower soil fertility base (e.g. Wakelin, van Koten, et al. Citation2013). As the productive capacity of these systems is limited by soil fertility, and particularly soil N content, the establishment of an effective biological nitrogen-fixing (BNF) legume component is considered essential to maintain the sector’s competiveness (Caradus et al. Citation1996).
While a range of legumes are used in New Zealand’s pastoral systems, white clover (Trifolium repens) remains the most important as phenotypically diverse cultivars are available that suit many grazing and management systems (Abberton Citation2006). However, the performance of white clover, and therefore BNF activity and the N fertility of the wider pasture, is dependent on the availability of sufficient populations of nodule forming (nod+) and nitrogen-fixing (nif+) Rhizobium leguminosarum bv. trifolii bacteria in soils (Sessitsch et al. Citation2002). These bacteria are not native to New Zealand, and the current soil populations are the historic result of inadvertent arrival on farm machinery or seed material, and deliberate inoculation of clover seed with commercial R. leguminosarum bv. trifolii stains (Lowther and Kerr Citation2011). As a result, the R. leguminosarum bv. trifolii in New Zealand soils vary immensely in population size, and importantly their symbiotic potential (i.e. their percentage effectives at fixing N2 when in symbiosis with a host legume). Indeed, surveys have found that the N2-fixation ability of rhizobia in New Zealand soils varies from just 2% effectiveness to >115% (Gaur and Lowther Citation1980; Lowther and Kerr Citation2011). As such, most pastures are performing well below average in regard to potential BNF, thus reducing the productive capacity and profitability of these farming systems.
In order to increase the BNF of New Zealand’s pastures, the establishment and maintenance of highly effective (BNF/symbiotic potential) R. leguminosarum bv. trifolii populations into farming soils are required. This is highly challenging, as pastoral soils often harbour a high background population of rhizobia. While these may have low symbiotic potential, they can be maintained over time by the host legume. Furthermore, the delivery of high titres of viable R. leguminosarum bv. trifolii into soils is challenging given the low survival of this species on clover seed, the preferred method of delivery (Lowther and Kerr Citation2011). In order to overcome these difficulties and build genetic gain in rhizobia populations in New Zealand’s pastures (and particularly the white clover symbiont, R. leguminosarum bv. trifolii), a large research programme is being supported by MBIE (C10X1308) and DairyNZ. As the overall aim is to ‘increase the performance of the rhizobia-legume symbiosis’, the research programme is inherently interdisciplinary in nature. The key goals are:
Isolate and identify the next generation of elite, N2 fixing R. leguminosarum bv. trifolii strains;
Create genetic markers for R. leguminosarum bv. trifolii to enable rapid selection of a range of desirable phenotypes, including tolerance to different soil pH conditions and environmental desiccation;
Characterise the potential for host (legume) genetics and physiological traits to control the selection of R. leguminosarum bv. trifolii from within genetically diverse soil populations;
Apply GIS and spatially explicit sampling strategies to identify soils and farming systems where inoculation of R. leguminosarum bv. trifolii may provide agronomic benefits;
Determine how to grow (ferment) and commercially deliver (formulate) high titres of viable and ‘fit-for-purpose’ R. leguminosarum bv. trifolii into pastoral soils.
It is through understanding the system-level ecology, spanning the plant × environment × R. leguminosarum bv. trifolii × microbiome (soil and plant) × management interactions, that new opportunities to farmers to build genetic gain in soil rhizobia populations will be identified. While advances in understanding interactions between rhizobia and edaphic properties of soils are providing useful knowledge (e.g. Drew et al. Citation2012), the nature and importance of interactions between rhizobia and the soil (or plant) microbiome remain unknown. Finally, the development of rapid and inexpensive molecular (DNA or other)-based detection systems for effective R. leguminosarum bv. trifolii in soils would be a significant step forward for applied rhizobia-legume outcomes not just for New Zealand, but globally.
Dairy farming: soil-borne disease suppression
The growth of forge plants is affected by a diverse range of pathogenic nematodes, fungi and oomycetes pathogens (Harvey and Harvey Citation2009). Historically, however, the control of these has been limited to chemical protection of seeds at planting; control of soil-borne diseases during the life of the pasture being too costly or difficult to warrant investment of resources beyond plant resistance breeding. However, as dairy remains generally more profitable than other forms of pastoral grazing, the extent and intensification of dairy farming in New Zealand have increased (Statistics New Zealand Citation2012), along with profit per ha (Ministry for Primary Industries Citation2017). When recently reassessed, the cost of soil-borne diseases to pasture production in dairy systems can now reach $1500 ha−1 y−1 (Wakelin, Eslami, et al. Citation2016). This finding has initiated new investigations into the control or management of pathogens and their diseases in pastures. However, control of soil-borne diseases is notoriously difficult to achieve, particularly in pasture-based systems where control needs to be durable for a number of years (Dignam et al. Citation2016). Furthermore, repeated inputs of synthetic chemicals (e.g. fungicides) neither provide enduring control (e.g. Bonanomi et al. Citation2010) nor are environmentally sustainable. As such, alternative methods for disease control need to be sought.
One of the most promising approaches is through management of soils to a disease-suppressive state. In this condition, the competitive activity and antagonistic suppression of the soil microbiota towards pathogens can reduce disease severity (Weller et al. Citation2002). In a number of instances, naturally disease-suppressive soils have been identified (Mazzola Citation2004), and in some instances, soils have been manipulated to induce a disease-suppressive state (Mazzola Citation2007; Raaijmakers and Mazzola Citation2016). However, this has not yet been achieved in New Zealand pastoral systems, and a number of significant hurdles must be overcome (Dignam et al. Citation2016). In particular, these include a shift in the traditional thinking of ‘species’ of bacteria or fungi being disease suppressive, to function-based understanding in which the ecology of traits such as production of antimicrobial agents or lytic enzymes underpins the disease suppression phenotype (Dignam et al. Citation2016). The management of the soil ecosystem towards this phenotype () will be reliant on environmental functional microarray technology (or similar) that can assess, in parallel, numerous functional genes from environmental DNA samples (e.g. GeoChip; He et al. Citation2007). When combined with new approaches to phenotype soils based on disease suppression (Dignam et al. Citation2015), and an understanding of the relationships and feedbacks between soil, environment, management and disease suppression (Wakelin, Cave, et al., Citation2016), significant gains in pastoral production and profitability maybe obtained. As with the example before, the microbiology within soils represents a vital natural resource that can be managed to protect plants from disease and sustainably increase ecosystem fertility (Azcón-Aguilar and Barea Citation1997).
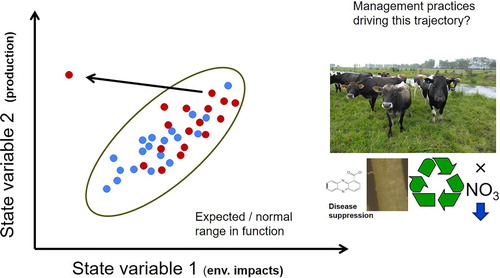
Figure 3. Defining a ‘normal operating range’ for a range of low input/intensity (blue dots), and high input/intensity (red dots) farming systems. Soil from each farm can be assessed for numerous phenotypes (functions) such as disease suppression, nutrient recycling, reduced nitrate leaching, effective rhizobia and mycorrhizal symbiosis, and increased carbon storage. As knowledge of how these vary among sites is accumulated, defining the normal (expected) operating range (NOR) of soils is possible. Soils with unique phenotypes can then be identified, for example, highly productive dairy systems with reduced rate of soil nitrate accumulation. By gaining an understanding of the edaphic and environmental drivers that underpin the state of each soil ecosystem, we can then begin managing farming systems to achieve multiple outcomes, and shifting soils from their NOR (direction and length of arrow on the plot indicating where and how far the system must shift).
Progressing towards multiple outcomes
Current efforts to manage soil biology for gains in agriculture, horticulture or forestry are typically based on single-function outcomes. In addition to the disease suppression and rhizobia examples above, these also include control of invertebrate pests (Zydenbos et al. Citation2016), reduction in nitrate leaching (Di and Cameron Citation2002), through to increased soil C storage (Schmidt et al. Citation2011). However, there is considerable and unrealised potential to achieve multiple outcomes across a wide number of soil functions () (Wakelin, Cave, et al., Citation2016). In this instance, management of soil microbial resources would involve deliberate strategies to shift the soil ecosystem from its ‘normal operating state’ to a more desirable multi-phenotype state. This is similar to breeding of livestock or plants for multiple traits. For soil ecosystems, however, we cannot directly visualise the phenotypes we are selecting for.
Ecological genomics
Clearly building genetic gain in a soil ecosystem is a challenging task. Not only is the ecosystem incredibly diverse in species, but it varies both spatially and temporally in ways that differ from those based on macroecological theory (Fierer et al. Citation2011; Hanson et al. Citation2012). Furthermore, functions in soil ecosystems are not often supported in a linear relationship with the diversity present, can be sensitive to effects and influence of other functions (e.g. the C–N cycling example earlier), and are often an ‘emergent outcome’ of multiple interactions among several functions. Against all this, how do we manage soil ecosystem for multiple outcomes?
An emerging tool to characterise the state of soil microbiology is ‘ecological genomics’. This refers to the analysis of DNA or RNA samples extracted directly from soil ecosystems, and thereby containing the genetic diversity and potential of the collective species present (Myrold et al. Citation2014; Nannipieri et al. Citation2014; Nesme et al. Citation2016). A particular issue is the massive amount of DNA present in the soil metagenome. A single gram of soil is likely to have >1000 Gbp of microbial DNA, compared with ‘just’ 3 Gbp in the human genome (Vogel et al. Citation2009). As such, complete sequencing of the soil metagenome remains a formidable challenge. However, technological advances in DNA and RNA (metatranscriptome) sequencing and analysis are providing new insights into the diversity and quantitative assessment of genes expressed within a soil ecosystem (Wakelin, Cave, et al., Citation2016). This emerging technology platform for applied environmental biogeochemistry provides a quantum step for functional assessment of microbial communities in relation to soil processes. This is particularly important for productive land uses such as agriculture and forestry, where the aim is specifically to understand the factors that drive dynamical feedback between plants, soil biota and soil function for both productive and environmental endpoints. Given the complexity of processes that collectively interact to effect soil functions, analytical tools based on complex systems analysis, such as functional molecular ecological network analysis (fMENA) (Deng et al. Citation2012), are being applied to determine key points of interdependencies among functions. For example, this approach could be used to determine the critical points where cycling of carbon is coupled with the nitrogen cycle and affects the rate of nitrate accumulation in soil. From this, we may reveal hitherto unknown opportunities to moderate nitrogen flow through soil ecosystems based on qualitative or quantitate management of carbon supply.
Conclusion
There are clearly multiple opportunities to manage soil microbiology to achieve productive and environmental outcomes for agriculture, horticulture and forestry. Ecological genomics, already being applied for human, animal and plant microbiome analyses, provides a destructive technology platform for making similar gains in soil ecosystems. Given the technology is recently emerging, researchers and land managers alike should be cognizant of Amara’s law: ‘we tend to overestimate the effect of a technology in the short run and underestimate the effect in the long run’. Thus, a long-term and pragmatic approach should be considered towards investment in soil ecological genomics for the land-based sectors. Furthermore, ‘sequencing everything’, without collection of metadata that effectively describes the ecosystem phenotype, constitutes a costly and resource-consuming exercise that achieves relatively little in building genetic gain in soil ecosystems. Research needs to be undertaken alongside farmers, land managers and the wider stakeholders to ensure that outcomes meet sector needs.
Acknowledgements
We thank the reviewers for their support and useful comments, and the editor for his assistance in preparing this work for publication.
Disclosure statement
No potential conflict of interest was reported by the author.
ORCID
Steven A. Wakelin http://orcid.org/0000-0002-1167-8699
Additional information
Funding
References
- Abberton MT, Fothergill M, Collins RP, Marshall AH. 2006. Breeding forage legumes for sustainable and profitable farming systems. Aspects of Applied Biology. 80:81–87.
- Andrews M, Edwards GR, Ridgway HJ, Cameron KC, Di HJ, Raven JA. 2011. Positive plant microbial interactions in perennial ryegrass dairy pasture systems. Annals of Applied BiologyVolume. 159:79–92. doi: 10.1111/j.1744-7348.2011.00473.x
- Angel R, Claus P, Conrad R. 2012. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. The ISME Journal. 6:847–862. doi: 10.1038/ismej.2011.141
- Angel R, Soares MIM, Ungar ED, Gillor O. 2010. Biogeography of soil archaea and bacteria along a steep precipitation gradient. The ISME Journal. 4:553–563. doi: 10.1038/ismej.2009.136
- Azarbad H, van Gestel CAM, Niklińska M, Laskowski R, Röling WFM, van Straalen NM. 2016. Resilience of soil microbial communities to metals and additional stressors: DNA-based approaches for assessing ‘stress-on-stress’ responses. International Journal of Molecular Sciences. 17:933. http://doi.org/10.3390/ijms17060933.
- Azcón-Aguilar C, Barea JM. 1997. Arbuscular mycorrhizas and biological control of soil-borne plant pathogens – an overview of the mechanisms involved. Mycorrhiza. 6:457–464. doi: 10.1007/s005720050147
- Balser TC, Firestone MK. 2005. Linking microbial community composition and soil processes in a Californian annual grassland and mixed-conifer forest. Biogeochemistry. 73:395–415. doi: 10.1007/s10533-004-0372-y
- Bates ST, Berg-Lyons D, Caporaso JG, Walters WA, Knight R, Fierer N. 2011. Examining the global distribution of dominant archaeal populations in soil. The ISME Journal. 5:908–917. doi: 10.1038/ismej.2010.171
- Bates ST, Clemente JC, Flores GE, Walters WA, Parfrey LW, Knight R, Fierer N. 2012. Global biogeography of highly diverse protistan communities in soil. The ISME Journal. 7:652–659. doi: 10.1038/ismej.2012.147
- Bardgett RD, van der Putten WH. 2014. Belowground biodiversity and ecosystem functioning. Nature. 515:505–511. doi: 10.1038/nature13855
- Beare MH, Hu S, Coleman DC, Hendrix PF. 1997. Influences of mycelial fungi on soil aggregation and organic matter storage in conventional and no-tillage systems. Applied Soil Ecology. 5:211–219. doi: 10.1016/S0929-1393(96)00142-4
- Bintrim SB, Donohue TJ, Handelsman J, Roberts GP, Goodman RM. 1997. Molecular phylogeny of archaea from soil. Proceedings of the National Academy of Sciences USA. 94:277–282. doi: 10.1073/pnas.94.1.277
- Bissett A, Richardson AE, Baker G, Wakelin S, Thrall PH. 2010. Life history determines biogeographical patterns of soil bacterial communities over multiple spatial scales. Molecular Ecology. 19:4315–4327. doi: 10.1111/j.1365-294X.2010.04804.x
- Bonanomi G, Antignani V, Capodilupo M, Scala F. 2010. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biology and Biochemistry 42:136–144. doi: 10.1016/j.soilbio.2009.10.012
- Bossio DA, Scow KM, Gunapala N, Graham KJ. 1998. Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microbial Ecology. 1:1–12. doi: 10.1007/s002489900087
- Bowatte S, Brock S, Newton PCD. 2009. Detection of ammonia oxidising archaea (AOA) in New Zealand soils. New Zealand Journal of Agricultural Research. 52:179–183. doi: 10.1080/00288230909510502
- Brevik EC, Sauer TJ. 2015. The past, present, and future of soils and human health studies. Soil 1, 35–46. doi:10.5194/soil-1-35-2015.
- Buckley DH, Graber JR, Schmidt TM. 1998. Phylogenetic analysis of nonthermophilic members of the kingdom Crenarchaeota and their diversity and abundance in soils. Applied and Environmental Microbiology. 64:4333–4339.
- Bulgarelli D, Rott M, Schlaeppi K, van Themaat EVL, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, et al. 2012. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 488:7409. doi: 10.1038/nature11336
- Bünemann EK, Schwenke GD, Van Zwieten L. 2006. Impact of agricultural inputs on soil microorganisms – a review. Australian Journal of Soil Research. 44:379–406. doi: 10.1071/SR05125
- Cao P, Zhang LM, Zheng YM, Di HJ, He JZ. 2012. Distribution and diversity of archaeal communities in selected Chinese soils. FEMS Microbiology Ecology. 80:146–158. doi: 10.1111/j.1574-6941.2011.01280.x
- Caradus JR, Woodfield DR, Stewart AV. 1996. Overview and vision for white clover. Grasslands Research and Practice Series. 6:1–6.
- Cavigelli MA, Robertson GP. 2000. The functional significance of denitrifier community composition in a terrestrial ecosystem. Ecology. 81:1402–1414. doi: 10.1890/0012-9658(2000)081[1402:TFSODC]2.0.CO;2
- Coleman DC, Whitman WB. 2005. Linking species richness, biodiversity and ecosystem function in soil systems. Pedobiologia. 49:479–497. doi: 10.1016/j.pedobi.2005.05.006
- Colloff MJ, Wakelin SA, Gomez D, Rogers SL. 2008. Detection of nitrogen cycle genes in soils for measuring the effects of changes in land use and management. Soil Biology and Biochemistry. 40:1637–1645. doi: 10.1016/j.soilbio.2008.01.019
- Crowther TW, Bradford MA. 2013. Thermal acclimation in widespread heterotrophic soil microbes. Ecology Letters. 16:469–4677. doi: 10.1111/ele.12069
- Curtis TP, Sloan WT, Scannell JW. 2002. Estimating prokaryotic diversity and its limits. Proceedings of the National Academy of Sciences USA. 99:10494–10499. doi: 10.1073/pnas.142680199
- Deng Y, Jiang Y-H, Yang Y, He Z, Luo F, Zhou J. 2012. Molecular ecological network analyses. BMC Bioinformatics. 13:113. doi: 10.1186/1471-2105-13-113
- Di H, Cameron KC. 2002. Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutrient Cycling in Agroecosystems. 64:237–256. doi: 10.1023/A:1021471531188
- Di H, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ. 2009. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geosciences. 2:621–624. doi: 10.1038/ngeo613
- Dignam BEA, O'Callaghan M, Condron LM, Raaijmakers JM, Kowalchuk GA, Wakelin SA. 2015. A bioassay to compare the disease suppressive capacity of pasture soils. New Zealand Plant Protection. 68:151–159.
- Dignam BEA, O'Callaghan M, Condron LM, Raaijmakers JM, Kowalchuk GA, Wakelin SA. 2016. Challenges and opportunities in harnessing soil disease suppressiveness for sustainable pasture production. Soil Biology and Biochemistry. 95:100–111. doi: 10.1016/j.soilbio.2015.12.006
- Dirzo R, Raven PH. 2003. Global state of biodiversity and loss. Annual Review of Environment and Resources. 28:137–167. doi: 10.1146/annurev.energy.28.050302.105532
- Drew EA, Denton MD, Sadras VO, Ballard RA. 2012. Agronomic and environmental drivers of population size and symbiotic performance of Rhizobium leguminosarum bv. viciae in Mediterranean-type environments. Crop and Pasture Sciences. 63:467–477. doi: 10.1071/CP12032
- Eilers KG, Lauber CL, Knight R, Fierer N. 2010. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biology & Biochemistry. 42:896–903. doi: 10.1016/j.soilbio.2010.02.003
- Fierer N. 2017. Embracing the unknown: disentangling the complexities of the soil microbiome. Nature Reviews Microbiology. 15:579–590. doi: 10.1038/nrmicro.2017.87
- Fierer N, Bradford MA, Jackson RB. 2007. Toward an ecological classification of soil bacteria. Ecology. 88:1354–1364. doi: 10.1890/05-1839
- Fierer N, Jackson RB. 2006. The diversity and biogeography of soil bacterial communities. Proceedings of the National Academy of Sciences USA. 103:626–631. doi: 10.1073/pnas.0507535103
- Fierer N, McCain CM, Meir P, Zimmermann M, Rapp JM, Silman MR, et al. 2011. Microbes do not follow the elevational diversity patterns of plants and animals. Ecology. 92:797–804. doi: 10.1890/10-1170.1
- Focht DD, Verstraete W. 1977. Biochemical ecology of nitrification and denitrification. Advances in Microbial Ecology. 1:134–214.
- Garbeva P, van Veen JA, van Elsas JD. 2004. Microbial diversity in soil: selection of microbial populations by plant and soil type. Annual Review of Phytopathology. 42:243–270. doi: 10.1146/annurev.phyto.42.012604.135455
- Garrett SD. 1963. Soil fungi and soil fertility. London: Pergamon Press Ltd.
- Gaur YD, Lowther WL. 1980. Distribution, symbiotic effectiveness, and fluorescent antibody reaction of naturalised populations of Rhizobium trifolii in Otago soils. New Zealand Journal of Agricultural Research. 23:529–532. doi: 10.1080/00288233.1980.10417878
- Giller PS. 1996. The diversity of soil communities, the ‘poor man’s tropical rainforest’. Biodiversity and Conservation. 5:135–168. doi: 10.1007/BF00055827
- Hannula SE, de Boer W, van Veen J. 2012. A 3-year study reveals that plant growth stage, season and field site affect soil fungal communities while cultivar and GM-trait have minor effects. PLoS ONE. 7:e33819. doi: 10.1371/journal.pone.0033819
- Hanson CA, Fuhrman JA, Horner-Devine MC, Martiny JBH. 2012. Beyond biogeographic patterns: processes shaping the microbial landscape. Nature Reviews Microbiology. 10:497–506. doi: 10.1038/nrmicro2795
- Harris K, Young IM, Gilligan CA, Otten W, Ritz K. 2003. Effect of bulk density on the spatial organisation of the fungus Rhizoctonia solani in soil. FEMS Microbiology Ecology. 44:45–56. doi: 10.1111/j.1574-6941.2003.tb01089.x
- Harvey IC, Harvey BM. 2009. Pasture diseases in New Zealand. Lincoln: Bio-Protection Research Centre; p. 144.
- He Z, Gentry TJ, Schadt CW, Wu L, Liebich J, Chong SC, Huang Z, Wu W, Gu B, Jardine P, et al. 2007. Geochip: a comprehensive microarray for investigating biogeochemical, ecological and environmental processes. The ISME Journal. 1:67–77. doi: 10.1038/ismej.2007.2
- Hendrix PF, Parmelee RW, Crossley DA, Coleman DC, Odum EP, Groffman PM. 1986. Detritus food webs in conventional and non-till agroecosystems. BioScience. 36:374–380. doi: 10.2307/1310259
- Henriksen TM, Breland TA. 1999. Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil Biology and Biochemistry. 31:1121–1134. doi: 10.1016/S0038-0717(99)00030-9
- Jackobsen I, Abbott LK, Robson AD. 1992. External hyphae of vesicular arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytologist. 120:371–380. doi: 10.1111/j.1469-8137.1992.tb01077.x
- Jangid K, Williams MA, Franzluebers AJ, Schmidt TM, Coleman DC, Whiteman WB. 2011. Land-use history has a stronger impact on soil microbial community composition than above ground vegetation and soil properties. Soil Biology & Biochemistry. 43:2184–2193. doi: 10.1016/j.soilbio.2011.06.022
- Jansa J, Mozafar A, Anken T, Ruh R, Sanders IR, Frossard E. 2002. Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza. 12:225–234. doi: 10.1007/s00572-002-0163-z
- Jenkinson DS. 1977. The soil microbial biomass. New Zealand Soil News. 25:213–218.
- Klemm D, Heublein B, Fink H-P, Bohn A. 2005. Cellulose: fascinating biopolymer and sustainable raw material. Angewandte Chemie International Edition. 44:3358–3393. doi: 10.1002/anie.200460587
- Kowalchuk GA, Stephen JR. 2001. Ammonia-oxidising bacteria: a model for molecular microbial ecological research. Annual Review of Microbiology. 55:485–529. doi: 10.1146/annurev.micro.55.1.485
- Kucey RMN. 1983. Phosphate-solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Canadian Journal of Soil Science. 63:671–678. doi: 10.4141/cjss83-068
- Kuramae EE, Yergeau E, Wong LC, Pijl AS, van Veen JA, Kowalchuk GA. 2012. Soil characteristics more strongly influence soil bacterial communities than land-use type. FEMS Microbiology Ecology. 79:12–24. doi: 10.1111/j.1574-6941.2011.01192.x
- Lauber CL, Hamady M, Knight R, Fierer N. 2009. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Applied and Environmental Microbiology. 75:5111–5120. doi: 10.1128/AEM.00335-09
- Lauber CL, Strickland MS, Bradford MA, Fierer N. 2008. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology and Biochemistry. 40:2407–2415. doi: 10.1016/j.soilbio.2008.05.021
- Laughlin RJ, Stevens RJ. 2002. Evidence for fungal dominance of denitrification and codenitrification in a grassland soil. Soil Science Society of America Journal. 66:1540–1548. doi: 10.2136/sssaj2002.1540
- Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 442:806–809. doi: 10.1038/nature04983
- Lowther WL, Kerr GA. 2011. White clover seed inoculation and coating in New Zealand. Proceeding of the New Zealand Grasslands Association. 73:93–102.
- Mander C, Wakelin S, Young S, Condron L, O'Callaghan M. 2012. Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biology & Biochemistry. 44:93–101. doi: 10.1016/j.soilbio.2011.09.009
- Marschner H, Dell B. 1994. Nutrient uptake in mycorrhizal symbiosis. Plant and Soil. 159:89–102. doi: 10.1007/BF00000098
- Marschner P, Yang CH, Lieberei R, Crowley DE. 2001. Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biology & Biochemistry. 33:1437–1445. doi: 10.1016/S0038-0717(01)00052-9
- Mazzola M. 2004. Assessment and management of soil microbial community structure for disease suppression. Annual Review of Phytopathology. 42:35–59. doi: 10.1146/annurev.phyto.42.040803.140408
- Mazzola M. 2007. Manipulation of rhizosphere bacterial communities to induce suppressive soils. Journal of Nematology 39:213–220.
- Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JHM, Piceno YM, DeSantis TZ, Andersen GL, Bakker PAHM, Raaijmakers JM. 2011. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100. doi: 10.1126/science.1203980
- Mertens J, Broos K, Wakelin SA, Kowalchuk GA, Springael D, Smolders E. 2009. Bacteria, not archaea, restore nitrification in a zinc-contaminated soil. The ISME Journal. 3:916–923. doi: 10.1038/ismej.2009.39
- Millard P, Singh BK. 2010. Does grassland vegetation drive soil microbial diversity? Nutrient Cycling in Agroecosystems. 88:147–158. doi: 10.1007/s10705-009-9314-3
- Ministry for Primary Industries 2017 Mar. Situation and outlook for primary industries. Wellington; 30 p.
- Myrold DD, Zeglin LH, Jansson JK. 2014. The potential of metagenomic approaches for understanding soil microbial processes. Soil Science Society of America Journal. 78:3–10. doi: 10.2136/sssaj2013.07.0287dgs
- Nannipieri P, Pietramellara G, Renella G. 2014. Omics in soil science. Norfolk: Caister Academic Press; 198 p.
- Nesme J, Achouak W, Agathos S, Bailey M, Baldrian P, Brunel D, Frostegard A, Heulin T, Jansson JK, Jurkevitch E, et al. 2016. Back to the future of soil metagenomics. Frontiers in Microbiology. 7:533. doi: 10.3389/fmicb.2016.00073
- Nicol GW, Glover A, Prosser JI. 2003. Molecular analysis of methanogenic archaeal communities in managed and natural upland pasture soils. Global Change Biology. 9:1451–1457. doi: 10.1046/j.1365-2486.2003.00673.x
- Pester M, Bittner N, Deevong P, Wagner M, Loy A. 2010. A ‘rare biosphere’ microorganism contributes to sulfate reduction in a peatland. The ISME Journal. 4:1591–1602. doi: 10.1038/ismej.2010.75
- Philippot L, Spor A, Hénault C, Bru D, Bizouard F, Jones CM, Sarr A, Maron P-A. 2013. Loss in microbial diversity affects nitrogen cycling in soil. The ISME Journal. 7:1609–1619. doi: 10.1038/ismej.2013.34
- Raaijmakers JM, Mazzola M. 2016. Soil immune responses. Science. 352:1392–1393. doi: 10.1126/science.aaf3252
- Reith F, Brugger J, Zammit CM, Gregg AL, Goldfarb KC, Andersen GL, DeSantis TZ, Piceno YM, Brodie EL, Lu Z, et al. 2012. Influence of geogenic factors on microbial communities in metallogenic Australian soils. The ISME Journal. 6:2107–2118. doi: 10.1038/ismej.2012.48
- Robertson GP, Klingensmith KM, Klug MJ, Paul EA, Crum JR, Ellis BG. 1997. Soil resources, microbial activity, and primary production across an agricultural ecosystem. Ecological Applications. 7:158–170. doi: 10.1890/1051-0761(1997)007[0158:SRMAAP]2.0.CO;2
- Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N. 2010. Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME Journal. 4:1340–1351. doi: 10.1038/ismej.2010.58
- Sessitsch A, Howieson JG, Perret X, Antoun H, Martínez-Romero E. 2002. Advances in rhizobium research. Critical Reviews in Plant Science. 21:323–378. doi: 10.1080/0735-260291044278
- Schimel JP, Gulledge J. 1998. Microbial community structure and global trace gases. Global Change Biology. 4:74–758. doi: 10.1046/j.1365-2486.1998.00195.x
- Schimel JP, Schaeffer SM. 2012. Microbial control over carbon cycling in soil. Frontiers in Microbiology. 3: Article 348. doi: 10.3389/fmicb.2012.00348
- Schloss PD, Handelsman J. 2006. Toward a census of bacteria in soil. PLoS Computational Biology. 2:e92. doi: 10.1371/journal.pcbi.0020092
- Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC, et al. 2011. Persistence of soil organic matter as an ecosystem property. Nature. 478:49–56. doi: 10.1038/nature10386
- Shoun H, Kim D-H, Uchiyama H, Sugiyama J. 1992. Denitrification by fungi. FEMS Microbiology Letters. 94:277–282. doi: 10.1111/j.1574-6968.1992.tb05331.x
- Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G. 2001. Bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Applied and Environmental Microbiology. 67:4742–4751. doi: 10.1128/AEM.67.10.4742-4751.2001
- Statistics New Zealand. 2012 Jun 30. Agricultural areas in hectares, by usage and region. http://www.stats.govt.nz/browse_for_stats/industry_sectors/agriculture-horticulture-forestry/2012-agricultural-census-tables/land-use.aspx.
- Steffan JJ, Brevik EC, Burgess LC, Cerdà A. 2017. The effect of soil on human health: an overview. European Journal of Soil Science. doi:10.1111/ejss.12451.
- Strickland MS, Lauber C, Fierer N, Bradford MA. 2009. Testing the functional significance of microbial community composition. Ecology. 90:441–451. doi: 10.1890/08-0296.1
- Štursová M, Žifčáková L, Leigh MB, Burgess R, Baldrian P. 2012. Cellulose utilization in forest litter and soil: identification of bacterial and fungal decomposers. FEMS Microbiology Ecology. 80:735–746. doi: 10.1111/j.1574-6941.2012.01343.x
- Torsvik V, Øvreås L, Thingstad TF. 2002. Prokaryotic diversity – magnitude, dynamics, and controlling factors. Science. 296:1064–1066. doi: 10.1126/science.1071698
- Ueda T, Suga Y, Matsuguchi T. 1995. Molecular phylogenetic analysis of a soil microbial community in a soybean field. European Journal of Soil Science. 46:415–421. doi: 10.1111/j.1365-2389.1995.tb01337.x
- Valentine DL. 2007. Adaptations to energy stress dictate the ecology and evolution of archaea. Nature Reviews Microbiology. 5:316–323. doi: 10.1038/nrmicro1619
- van Straalen NM, Roelofs D. 2006. An introduction to ecological genomics. Oxford University Press; p. 320.
- Vogel TM, Simonet P, Jansson JK, Hirsch PR, Tiedje JM, van Elsas JD, Bailey MJ, Nalin R, Philippot L. 2009. Terragenome: a consortium for the sequencing of a soil metagenome. Nature Reviews Microbiology. 7:252. doi: 10.1038/nrmicro2119
- Wakelin SA, Barratt BIP, Gerard E, Gregg AL, Brodie EL, Andersen GL, DeSantis TZ, Zhou J, He Z, Kowalchuk GA, O’Callaghan M. 2013. Shifts in the phylogenetic structure and functional capacity of soil microbial communities follow alteration of native tussock grassland ecosystems. Soil Biology & Biochemistry. 57:675–682. doi: 10.1016/j.soilbio.2012.07.003
- Wakelin SA, Cave VM, Dignam BE, D’Ath C, Tourna M, Condron LM, Zhou J, Nostrand JD V, O’Callaghan M. 2016. Analysis of soil eDNA functional genes: potential to increase profitability and sustainability of pastoral agriculture. New Zealand Journal of Agricultural Research. 59:333–350. doi: 10.1080/00288233.2016.1209529
- Wakelin SA, Colloff MJ, Harvey PR, Marschner P, Gregg AL, Rogers SL. 2007. The effects of stubble retention and nitrogen application on soil microbial community structure and functional gene abundance under irrigated maize. FEMS Microbiology Ecology. 59:661–670. doi: 10.1111/j.1574-6941.2006.00235.x
- Wakelin S, Eslami Y, Dake K, Dignam B, O’Callaghan M. 2016. Cost of root disease on white clover growth in New Zealand dairy pastures. Australasian Plant Pathology. 45:289–296. doi: 10.1007/s13313-016-0411-x
- Wakelin SA, Gregg AL, Simpson RJ, Li GD, Riley IT, McKay AC. 2009. Pasture management clearly affects soil microbial community structure and N-cycling bacteria. Pedobiologia. 52:237–251. doi: 10.1016/j.pedobi.2008.10.001
- Wakelin SA, MacDonald LM, O’Callaghan M, Forrester ST, Condron LM. 2013. Soil functional resistance and stability are linked to different ecosystem properties. Austral Ecology. 39:522–531. doi: 10.1111/aec.12112
- Wakelin SA, Macdonald LM, Rogers SL, Gregg AL, Bolger TP, Baldock JA. 2008. Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biology and Biochemistry. 40:803–813. doi: 10.1016/j.soilbio.2007.10.015
- Wakelin SA, van Koten C, O’Callaghan M, Brown M. 2013. Physicochemical properties of 50 New Zealand pasture soils: a starting point for assessing and managing soil microbial resources. New Zealand Journal of Agricultural Research. 56:248–260. doi: 10.1080/00288233.2013.822003
- Waldrop MP, Balser TC, Firestone MK. 2000. Linking microbial community composition to function in a tropical soil. Soil Biology & Biochemistry. 32:1837–1846. doi: 10.1016/S0038-0717(00)00157-7
- Weller DM, Raaijmakers JM, McSpadden Gardener BB, Thomashow LS. 2002. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annual Review of Phytopathology. 40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010
- Wheeler KA, Hurdman BF, Pitt JI. 1991. Influence of pH on the growth of some toxigenic species of Aspergillus, Penicillium and Fusarium. International Journal of Food Microbiology. 12:141–150. doi: 10.1016/0168-1605(91)90063-U
- Whitelaw MA. 2000. Growth promotion of plants inoculated with phosphate solubilizing fungi. Advances in Agronomy. 69:99–151. doi: 10.1016/S0065-2113(08)60948-7
- Zak DR, Pregitzer KS, Burton AJ, Edwards IP, Kellner H. 2011. Microbial response to a changing environment: implications for the future functioning of terrestrial ecosystems. Fungal Ecology. 4:386–395. doi: 10.1016/j.funeco.2011.04.001
- Zydenbos S, Townsend RJ, Lane PMS, Mansfield S, O’Callaghan M, van Koten C, Jackson TA. 2016. Effect of Serratia entomophila and diazinon applied with seed against grass grub populations on the North Island volcanic plateau. New Zealand Plant Protection. 69:86–93.
