 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Asparagopsis (A. taxiformis and A. armata) as a dry feed additive, and novel oil-based formulations containing bromoform are effective at reducing enteric methane emissions in ruminant livestock. An inclusion rate of Asparagopsis at 0.2%–0.5% of the daily diet for cattle of say 470 kg consuming 15 kg dry matter (DM) per day is equivalent to 30–75 g/day Asparagopsis delivering 180–450 mg/day bromoform (at 6 mg bromoform per gram of seaweed) per animal equivalent to 0.4–1.0 mg/kg/day of bromoform. This is a low dose when compared to those evaluated in animal toxicity studies. A response relationship in terms of emission reductions and the dosage of bromoform ingested, is established, as opposed to dose responses linked to seaweed biomass. Most of the research studies on bromoform as a methane mitigant have used Asparagopsis. Nevertheless, the primary interest from a regulatory perspective is bromoform, the most prevalent bioactive. Regulatory considerations to enable safe use of bromoform for methane mitigation in ruminants are described. A key conclusion is that bromoform, administered in very low doses, is not bioavailable at measurable levels. Hence the risk of residue transfer or toxicity in livestock and humans will be minimal.
Introduction
Asparagopsis taxiformis was shown to be the most promising in an in vitro screening process to identify the potential of different seaweeds to reduce methane synthesis (Machado et al. Citation2014). Recent research with animals has shown that the seaweed Asparagopsis (both A. taxiformis and A. armata) is effective at reducing methane emissions when included in the diet of ruminant livestock (Li et al. Citation2018; Roque et al. Citation2019; Kinley et al. Citation2020; Lean et al. Citation2021; Roque et al. Citation2021; Stefenoni et al. Citation2021; Fennessy et al. Citationin preparation). Naturally occurring bromoform in Asparagopsis is considered responsible for this effect and oil-based formulations containing Asparagopsis-derived bromoform included in the diet have been shown to be as effective as the seaweed itself (Alvarez-Hess et al. Citation2023). Earlier publications have noted that the concentration of bromoform is crucial in determining the effectiveness of Asparagopsis and should remain above 1 mg bromoform/g organic matter (Vucko et al. Citation2017) in an in vitro study, and that methane emissions can be reduced incrementally by increasing the percentage of Asparagopsis in the diet (Roque et al. Citation2019; Kinley et al. Citation2020; Roque et al. Citation2021).
In this review, dose–response relationships between the reduction in methane and the dosage of bromoform (mg) ingested from seaweed (or from bromoform in an oil-based delivery vehicle) as part of the diet for both sheep and cattle are elucidated, as opposed to dose responses linked to seaweed biomass. This complements recent in vitro research (Andreen et al. Citation2023). Although most of the research studies completed to date, on bromoform as a methane mitigant have used Asparagopsis, as a source of bromoform, (with one including bromoform in an oil, extracted from Asparagopsis), the primary interest from a regulatory perspective is on bromoform, but in this review, we consider the issues from both an Asparagopsis and a bromoform perspective.
A number of authors offer different perspectives on the opportunities and challenges faced in the development of Asparagopsis for methane suppression, including efficacy, cost-effectiveness and safety concerns (Abbott et al. Citation2020; Munoz-Tamayo et al. Citation2021; Vaskoska Citation2021; Wheeler et al. Citation2021; Glasson et al. Citation2022; Jia et al. Citation2022; Roskam et al. Citation2022; Ungerfeld Citation2022; Ungerfeld et al. Citation2022; De Bhowmick and Hayes Citation2023; Firkins and Mitchell Citation2023). In this respect, the toxicity of bromoform is cited as a reason for researching the potential of northern hemisphere seaweeds (Abbott et al. Citation2020; Roskam et al. Citation2022) as alternatives to Asparagopsis.
This publication does not address many of the issues raised by these authors, such as questions on the commercial viability of Asparagopsis farming (Wheeler et al. Citation2021) or aspects relating to seaweeds and atmospheric health (see Glasson et al. Citation2022) which are beyond the scope of this review. However, conflicting views, noted by De Bhowmick and Hayes (Citation2023), on health and residue impacts of Asparagopsis or bromoform when used in amounts that mitigate methane production are addressed.
Vaskoska (Citation2021) defines two important health related questions, relevant to this review, that would arise from the commercial use of bromoform for ruminants: (1) is bromoform likely to end up in the food chain? (2) could there be a carcinogenic risk in humans through consumption of milk and meat if bromoform gets transferred from the gastro-intestinal tract to the milk or meat? Concerns relating to bromoform apply to consideration of Asparagopsis as a source of bromoform, oil-based formulations containing Asparagopsis-derived bromoform or to scenarios where synthetic bromoform delivered as a feed additive or in a slow-delivery intra-ruminal bolus.
In relation to the first question, bromoform is already present in the food chain from a variety of different sources. The more pertinent question is whether bromoform when delivered, in an appropriate manner, is absorbed, and if concentrations in the body exceed the low levels that are likely to be present from a range of natural and anthropogenic sources (Glasson et al. Citation2022) and see Sections 2–4 of this paper. The second question on toxicology suggests a lack of awareness by several authors (e.g. Abbott et al. (Citation2020); Roskam et al. (Citation2022)) of the basic principles of toxicology, namely that all things are poisonous, and dose dictates toxicity or safety, and that bioavailability, which is the proportion of a substance which enters the circulation when introduced into the body and so is able to have an active effect, is a prerequisite for systemic effects (Casarett and Doull Citation2013; Glasson et al. Citation2022; Timbrell and Barile Citation2023).
It is apparent from several recent publications (Abbott et al. Citation2020; Vaskoska Citation2021; Wheeler et al. Citation2021; Roskam et al. Citation2022; Ungerfeld Citation2022; Ungerfeld et al. Citation2022; De Bhowmick and Hayes Citation2023) that there is a lack of clarity, as to the relevance of bromoform toxicity in laboratory animal studies. In this respect (Glasson et al. Citation2022) provided a science-based approach to elucidating the toxicology of bromoform. Because of this confusion, and the continued highlighting of toxicology issues and conflicting views (De Bhowmick and Hayes Citation2023), we delve further than before into fundamental principles of toxicology linked to bromoform exposure pathways and risk; this includes a comprehensive assessment of the relevance of toxic effects, defined in laboratory animal studies, and their relevance to livestock and human health (see Sections 5 and 6).
Given that the available data relate to the use of Asparagopsis as a source of bromoform, our assessments focus on formats where Asparagopsis or an oil-based derivative are fed to livestock as part of the diet. The delivery format is important, as it can be expected to impact the release within the reticulo-rumen (rumen) and hence the pharmacokinetics of bromoform (Glasson et al. Citation2022). For any particular format, the specific issue is understanding the potential for absorption of bromoform into the systemic circulation of the animal. The key emerging principle is that a delivery format that allows a sustained low-level release of bromoform, whether of natural or synthetic origin, is likely to be required to ensure that bromoform is effective and retained within the rumen environment. Relevant principles and questions on residues, and considerations regarding the food-chain and toxicology are addressed in later sections of this paper; these form the basis for recommendations regarding the safe use of Asparagopsis and bromoform, at the low levels required for methanogensis mitigation in ruminants.
In summary, this review covers advances in methane suppression, natural and anthropogenic bromoform and potential dietary exposure scenarios, the risk to animals and specifically their health when fed bromoform and the risk to consumers from the transfer of bromoform from the ruminant diet to edible parts of the animal (carcass/muscle or organs such as liver, kidney or milk products). Regulatory approaches and the consideration of a MRL (Food Act 2014) for bromoform, regardless of its source, to address risk assessment criteria for food residues, are also explored in Section 7.
Section 1: Asparagopsis and methane mitigation – to dose-dependent suppression mediated by bromoform
Options for mitigation of methane emissions from livestock include selective breeding, vaccines that target the methanogens, changes in animal management and feed additives or other manipulations of the diet. Reductions in methane production in beef cattle have been reported for short-chain nitro-compounds (Dijkstra et al. Citation2018), synthetic halogenated compounds (Sawyer et al. Citation1974; Tomkins et al. Citation2009; Patra et al. Citation2017), and naturally synthesised halogenated compounds in seaweed (Kinley et al. Citation2020). In the latter case, interest has focused on red seaweeds with anti-methanogenic activity, particularly Asparagopsis species. These have the capacity to synthesise and store halogenated methane analogues, such as bromoform and dibromochloromethane, within specialised gland cells as a natural defence mechanism (Paul et al. Citation2006). These bromo-compounds are structural analogues of methane and are effective in reducing methane production, through competitive inhibition of methane biosynthesis (Liu et al. Citation2011). The evidence is that bromoform constitutes the vast majority of bromo-compounds present in Asparagopsis (Glasson et al. Citation2022).
We have summarised the results of all published studies and one unpublished study where animals were fed Asparagopsis, methane emissions were measured and the bromoform intake can be calculated; five trials met the criteria, three with cattle and two with sheep. The data and trial details are in .
Table 1. Summary of studies in ruminants fed AsparagopsisFootnotea with details of the studies, and the methane response (reduction) expressed per mg of bromoform.
As there were insufficient data to conduct a meta-analysis, we analysed the available data in a number of ways to ascertain the most appropriate form of expression. The mean effect of bromoform on methane reduction averaged −0.33 ± 0.15 (standard deviation) grams of methane per mg of bromoform consumed (n of 16 groups of animals). When the data are expressed as a dose–response by regression (), the equation (with the intercept constrained to zero) is:
(1)
(1)
Figure 1. The relationship between the reduction in methane emissions and the bromoform intake for five sets of data (the full data are in ).
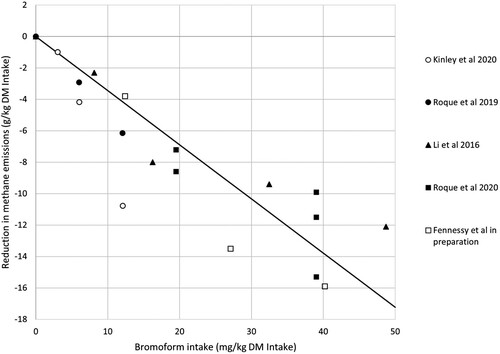
Thus, the regression coefficient of −0.25 grams of methane per mg of bromoform intake is slightly lower than the mean reduction of −0.33.
Given that the basal diet impacts the level of methane, the regression relationship between methane emissions (g methane per kg DMI) and bromoform intake is of interest. The regression relationship was:
(2)
(2) A comparison of the variances for the two regression relationships indicates that the reduction in methane as a function of bromoform intake provides a better estimate of the efficacy of bromoform as the 59% reduction in variance from 24.85 (4.992) in Equation (2) to 10.20 (3.192) in Equation (1) was considered meaningful. In summary, while there must be a limit to the absolute scale of the methane response in that the baseline control value sets the upper limit on any response, a linear regression provides a reasonable description of the relationship in the range of data sets assessed.
Given the differences in concentrations of bromoform in the product used, the reduction in methane emissions is expressed on a dosage of bromoform basis (per mg bromoform), to enable more direct comparisons to be made between the different trials (as per ).
In a 90-day study, beef cattle showed a 98% reduction in methane when A. taxiformis (6.6 mg bromoform/gram), was included at 0.33% of feed dry matter (DM) (Kinley et al. Citation2020); the average reduction over three dose rates ranged from −0.50 to −0.18 g of methane per mg of bromoform, with an apparent dose response to the level of Asparagopsis in the diet, independent of the bromoform content.
In a short 14-day study, lactating dairy cows showed a 20–43% reduction in methane when A. armata (1.3 mg bromoform/gram) was included at 0.92% or 1.84% of dry matter (DM), (Roque et al. Citation2019); the response was equivalent to 0.50 g reduction in methane per mg of bromoform; however, in this study, there were detrimental effects on feed intake.
In a 147-day study, beef cattle showed a 33–80% reduction in methane when A. taxiformis (7.8 mg bromoform/gram), was included at 0.45% or 0.83% of feed DM on three different diets (Roque et al. Citation2021); the response was equivalent to 0.15–0.25 g reduction in methane per mg of bromoform.
In a recent 16-day study with sheep fed A. armata (6.6 mg bromoform per gram of Asparagopsis dry matter fed once daily), Fennessy et al (Citationin preparation) found an average reduction in methane equivalent to 0.40 g per mg of bromoform; the sheep were fed 13–43 mg bromoform per kg dry matter intake; there was no effect on feed intake.
In a 27-day study in lactating cows with A. armata steeped in edible oil (ASP-Oil) to extract and stabilise bromoform, two formulations were tested – one with the Asparagopsis biomass included and one with biomass removed (Alvarez-Hess et al. Citation2023). They found reductions in methane equivalent to 0.63 g methane per mg bromoform.
Naturally occurring bromoform was deemed responsible for the beneficial effect on methane production. Importantly, the site of action is described as within the gut (rumen) and absorption of bromoform is neither needed nor desirable.
The results of these trials are consistent with the conclusion of Glasson et al. (Citation2022) namely that, use of the red seaweeds A. armata and A. taxifomis as dietary interventions for reducing enteric methane production shows strong promise. The Alvarez-Hess et al. (Citation2023) study takes this approach to a new level indicating that an oil-stabilised formulation of seaweed bromoform has potential if used in ways that deliver small amounts of bromoform safely.
Further analyses of these studies shows that an Asparagopsis inclusion rate of 0.2%–0.5% of the diet for cattle consuming 15 kg dry matter (DM) per day is equivalent to 30–75 gm/day Asparagopsis which will in turn deliver 180–450 mg/day bromoform per animal (based on a product containing 6 mg bromoform per gram). For cattle weighing 470 kg, the resultant dose of bromoform would equate to 0.4–1.0 mg/kg/day of bromoform, a relatively low dose when compared to doses evaluated in conventional animal toxicity and metabolism studies. Similar low doses have been delivered in novel oil-based formulations based on steeping Asparagopsis in oil and feeding the resultant product (Alvarez-Hess et al. Citation2023). These contained bromoform at 3.5 mg/g (ml) delivering approximately 480 mg/day bromoform to cows (average weight 578 kg), which equates to 0.83 mg/kg/day bromoform. The format appeared to be as effective as dried Asparagopsis at reducing methane emissions. In conclusion, analysis of the trials establishes a dose–response relationship in terms of reductions in methane emissions and the dose (mg) of bromoform ingested, which is relevant to any future methane emission mitigation strategies.
Section 2: Ensuring minimal residue risks to consumers in a pharmacokinetic context
Confirmation that bromoform residues are not transferred to edible tissues and offal to the detriment of food safety, or at concentrations above those background concentrations that result from both natural and anthropogenic sources, is important. In the context of any vehicle/format containing bromoform, and its application for methane suppression, the absorption or lack of absorption of bromoform from the gut will be influenced by the delivery format and the release characteristics. Key factors will include:
characteristics of the vehicle and the quantum of bromoform released from the vehicle per unit time,
the pattern including duration and rate of release of the bromoform from the vehicle, and
the rate of microbial uptake or breakdown of bromoform in the reticulo-rumen.
In this respect, the evidence to date shows the risk to consumers from the transfer of bromoform from Asparagopsis or oil-based formulations containing Asparagopsis or naturally derived bromoform, when used appropriately, is negligible. Nevertheless, given the importance of these consideration and related research findings, further details are provided below.
As previously noted, studies in livestock fed Asparagopsis in feed at amounts < 1% Asparagopsis (up to 40 mg bromoform/kg DM intake) show no or negligible residues of bromoform in milk, meat, fat or offal. In the study by Roque et al. (Citation2019), milk samples from control animals were also taken and the level of bromoform was 0.11 µg/L, with no statistical difference between the control and either dosing group. In another study (Stefenoni et al. Citation2021), dairy cattle were fed 0.25% and 0.5% of the diet as Asparagopsis (specific bromoform concentration was not stated) for 28 days. Milk samples were taken from control and 0.5% dosing groups at the morning and afternoon milkings on days 23 and 24 of the study. As with the Roque et al. (Citation2019) study, bromoform was detected in the milk from control animals (16.5 µg/L, n = 4) and from animals in the 0.5% dosing group reported (28.9 µg/L, n = 4), but the difference was not statistically significant (p > 0.05).
Further, research to date has shown that feeding Asparagopsis containing bromoform at concentrations of up to 39 mg/kg diet DM to beef cattle for 147 days and up to 12 mg/kg diet DM to lactating cows or oil-based delivery of comparable amounts of bromoform for 27 days, did not result in measurable concentrations of bromoform in meat or milk over and above those commonly found as background levels in milk or drinking water (Roque et al. Citation2021; Alvarez-Hess et al. Citation2023). In the recent study by Alvarez-Hess et al. Citation2023 in lactating cows, a significant increase of bromoform in milk was reported (from 0.3 µg/l in controls to between 2 and 3 µg/l in the cows fed the oil-based formulations containing Asparagopsis-derived bromoform). However, these concentrations are similar to those found as background concentrations in water, milk and other food and beverages, and are far lower than the maximum acceptable values (MAV) for bromoform in drinking water for New Zealand of 100 µg/l.
This weight of evidence supports the conclusion that bromoform (at up to 1 mg/kg live weight) in Asparagopsis has low or no bioavailability when relatively small amounts of seaweed (0.2% to 0.5% of the daily diet) containing bromoform are fed in the diet of ruminants; similar outcomes were observed when oil-based formulations with Asparagopsis-derived bromoform at similar rates of bromoform were fed to cows. This indicates a lack of toxicologically-significant absorption of bromoform. The lack of bioavailability in ruminants at the minimum effective inclusion level is consistent with the bromoform from these formulations exerting its effect in the rumen and on its microbiota, and not systemically. Hence, reductions in methane emissions can occur without any of the systemic adverse health effects associated with bromoform. This reduction in emissions can also occur without the risk of the transfer of bromoform from the diet to the systemic circulation and hence to edible parts of the animal or milk products, at concentrations above those levels that naturally occur from other sources, such as drinking water. These conclusions are consistent with the comparatively small amounts of bromoform needed to produce an effect; in this respect, both dried Asparagopsis itself or an oil formulation have the capacity to provide a slow-release source of bromoform which allows for uptake by ruminal Archaea to induce an anti-methanogenic effect (Glasson et al. Citation2022) and, by inference (Loh et al. Citation2020), degradation within the rumen of any surplus.
The transfer of seaweed-derived bromoform to milk, urine, faeces, and animal tissue in cows has also been studied under unusual conditions (Muizelaar et al. Citation2021). The feeding rate of Asparagopsis to cattle was high but the daily dose of bromoform would have been comparable to other feeding trials (). The most striking features of the study were that animal husbandry appeared compromised, and that the dosing regimen with twice daily administration as a bolus in 2–4 litres of water, in fasted animals, prior to the normal feed, was changed during the trial. Dosing semi-fasted animals in this way could likely lead to unwanted absorption of bromoform, that would not occur normally when seaweed is eaten voluntarily as part of the diet in an appropriate regimen or vehicles as outlined in other more relevant studies (Li et al. Citation2018; Roque et al. Citation2019; Kinley et al. Citation2020; Roque et al. Citation2021; Stefenoni et al. Citation2021; Alvarez-Hess et al. Citation2023). Given the unusual circumstances, the results of this study are difficult to interpret, and it is not possible to assign any of the adverse effects reported to bromoform as meaningful and thus the residue results are questionable. Residues were reported in urine, which is surprising, and it is also difficult to understand how residues were detected in urine and not in faeces. Notably in laboratory studies, when absorption is induced following oral gavage of large doses of bromoform, only a small proportion of the dose was excreted in urine (see below).
Section 3: Pharmacokinetics at high doses in non-ruminant animals and its relevance
Section 2 focused on determining whether or not bromoform is absorbed following use of dried Asparagopsis and oil-based formulations as feed supplements, which is complemented by earlier metabolism research. In a different context, absorption, distribution, metabolism, and excretion (ADME) assessments on bromoform have been undertaken in laboratory animals at doses much higher than those required to inhibit methane production in ruminants (0.4–1.0 mg/kg/day of bromoform). Despite the debatable relevance of these laboratory studies because of the high doses used, they are summarised below for completeness. In addition, such studies contribute to our understanding of the likely ADME of bromoform should the pattern of bromoform release in the reticulo-rumen exceed the capacity of the microbes to take up the bromoform.
In rodent studies, at least 76% of a single dose (100 mg/kg) for rats and 62% of a single dose (150 mg/kg) for mice was absorbed following administration by oral gavage (Mink et al. Citation1986). When rats and mice were given these very high oral doses of bromoform, bromoform was absorbed but also quickly excreted with a half-life of 0.8 h in rats and 8 h in mice (Mink et al. Citation1986). Bromoform and its metabolites are excreted primarily through the lungs (Mink et al. Citation1986), with these authors showing that only 1–2% of a single gavage dose was retained in soft tissues of rats 8 h after dosing, consistent with the short half-life of bromoform. In another study, following an oral dose (2–16 mg bromoform/kg) to rats, the highest levels detected were in adipose tissue and blood (Parra et al. Citation1986). Lower concentrations were found in the kidneys, brain, and liver. The concentrations in the liver were below the detection limit after 1 h, presumably because metabolism of bromoform occurred rapidly.
Like many natural compounds or xenobiotics, bromoform is metabolised in the liver by cytochrome P450 oxidases; carbon monoxide and carbon dioxide are the primary products (Ahmed et al. Citation1977; Stevens and Anders Citation1979, Citation1981; ATSDR Citation1990). In rats and mice dosed with radiolabelled bromoform, elimination occurred primarily through the lungs in expired air within 8 h of dosing (Mink et al. Citation1986). Rats excreted approximately 4% of the total dose as CO2 and 70% as unmetabolized compound, while mice eliminated 40% as CO2 and 6% as unmetabolized bromoform, indicating that mice metabolise bromoform more extensively than rats. The urine of rats and mice contained <5% of the radiolabel after 8 h and <10% after 36-48 h. Lucas (Citation1928) reported that rabbits injected rectally with a bromoform/ olive oil mixture excreted 0.3% to 1.2% of the administered dose (as bromine) in the urine.
Relevance
For xenobiotics or natural compounds to produce systemic pharmacological or toxicological effects (i.e. affecting the whole body or an area distant from its entry point rather than a specific (local) area), absorption into the blood from the gut and distribution to target organs is a prerequisite. This did occur in the above cited rodent studies but the evidence from the livestock studies is that there is minimal systemic uptake of bromoform by animals ingesting bromoform in Asparagopsis. Hence the relevance of the rodent studies to livestock is highly debatable, because of the high doses used. Nevertheless, they do demonstrate that bromoform is not persistent in mammals, and when low levels of bromoform are absorbed from exposure to drinking water or other food sources, they will be readily metabolised and subsequent excretion will be rapid. Similarly, if there is absorption of small amounts of bromoform, over and above background levels, metabolism in the liver by P450 cytochrome oxidase and subsequent excretion of bromine and elimination of CO2 is likely to be very rapid, though this requires further confirmation in ruminants.
In conclusion, given the lack of detectable bromoform above background levels in livestock tissues or products and the rapid excretion of elevated bromoform, demonstrated in laboratory studies, it can be concluded that there is a very low risk to consumers. Importantly, background concentrations of bromoform in milk are similar to those recorded in milk from cows fed Asparagopsis or the recently developed oil-based formulation (Alvarez-Hess et al. Citation2023) and are well below WHO, NZ or USA standards for drinking water.
Section 4: Bromoform and potential dietary exposure scenarios in context
Whilst the studies outlined above lead to conclusions that appropriate delivery of bromoform should not result in human exposure to bromoform, information on other possible routes of dietary exposure are included in this section for completeness and to provide context. Bromoform and related compounds such as dibromochloromethane, as well as being biosynthesised naturally (e.g. present in seaweeds) may also form when chlorine reacts with other substances in water, such as decomposing plant material. They can also be readily synthesised in the laboratory and at commercial scale.
Both synthetic and natural bromoform have the same chemical structure (CHBr3). Synthetic bromoform is commonly described as a brominated organic solvent, related to chloroform. Historically, bromoform was used as a medicine in the early part of the last century as a sedative for children with whooping cough. Bromoform toxicity in humans and animals is a rare occurrence. Poisoning incidents have been associated with accidental overdoses mainly in children who were treated with bromoform (Du Plessis et al. Citation2020).
Both bromoform and dibromochloromethane are sometimes found in drinking water that has been chlorinated, and exposure can occur from inadvertent ingestion of water in swimming pools. The amount of bromoform and dibromochloromethane in drinking water can change considerably from day to day, depending on the source, temperature, amount of plant material in the water, amount of chlorine added, and a variety of other factors. Consumption of chlorinated drinking water, fresh water or desalinated seawater (Quivet et al. Citation2022) is the principal route of human exposure to bromoform. Water treatment with chlorine oxidises any bromide present to bromine, from which bromination reactions can occur with organic matter to form brominated by-products, including bromoform (MoH Citation2016). This reaction also occurs with ozone treatment of water that contains bromide and natural organic matter (MoH Citation2016). Bromoform has been detected in many drinking-water systems, both in samples collected at treatment facilities or along the distribution system and in samples collected from natural and untreated water sources. Concentrations in treated drinking-water typically range from < 1–10 µg/l (IARC Citation1991). In this respect, bromoform was detected in 12% of 881 drinking water samples through a New Zealand Ministry of Health survey programme (Nokes Citation1999; cited in On et al. Citation2008).
The maximum acceptable values (MAV) for bromoform in drinking water standards for New Zealand have been set at 100 µg/l, which aligns with the WHO recommended guideline (MoH Citation2016; WHO Citation2022). In the US, the MAV is set at 80 µg/l. Trihalomethanes found in drinking water include chloroform, bromodichloromethane, dibromochloromethane, and bromoform. In Europe, a total trihalomethane limit of 100 µg/l is specified for drinking water (EC Citation2020). Bromoform concentrations in drinking water in milk residue trials in ruminant livestock, described in an earlier section, have not been reported so it is not known how much of an influence this had on the residues measured.
It is also noteworthy that, in addition to exposure of humans and livestock through drinking water, low-level exposure to bromoform and dibromochloromethane will have occurred in the past and in recent times, from animals and humans eating Asparagopsis and from other exposure and dietary sources, including cleaning of food during processing or cleaning of processing equipment.
Chlorinated water, including municipal drinking water (Ryan et al. Citation2012) may be used in cleaning and rinsing of farm equipment and milking equipment. Bromoform and chloroform are amongst the most frequently used disinfectant by-products, providing a route for direct entry of bromoform into milk. An evaluation of chloroform (a similar trihalomethane to bromoform) formation in milking equipment identified that the use of chlorine-based disinfectants contributed to levels in milk (Ryan et al. Citation2012). Reported chloroform concentrations of up to 10 µg/l are comparable to bromoform residues reported in milk of control animals by Roque et al. (Citation2019) and Stefenoni et al. (Citation2021). Further, a study of wastewater effluent in the US associated with dairy facility input determined concentrations of bromoform in wastewaters of up to 0.95 µg/l (Hladik et al. Citation2016), which is 1% of the MAV.
In nature, these bromo-compounds occur naturally in a diverse range of microalgae (Shibazki et al. Citation2016; Sturges et al. Citation1992) and seaweeds including Ascophyllum nodosum, Fucus vesiculosis, Enteromorpha linza, Ulva lacia, Gigartina stellata (Gschwend et al. Citation1985), Laminariales laminaria (Class et al. Citation1986), and Macrocystis pyrifera (giant kelp) (Goodwin et al. Citation1997), as well the Asparagopsis species (Paul et al. Citation2006). In New Zealand, there is a wide range of red, brown and green seaweeds, including species known to produce bromoform such as A. armata, Macrocystis pyrifera and Ulva linza (Nelson et al. Citation2019), as well as many where this potential has not been studied. Seaweeds are also in use as feed and human dietary supplements, with a range of seaweed-based feeds available in New Zealand, including Macrocystis pyrifera. Similarly, seaweed-based fertilisers and tonics also see use in cropping and may be consumed in this form if grazing animals are introduced to freshly fertilised paddocks. Other dietary sources of bromoform are possible and organobromines are reported to be formed in terrestrial plants, including brassica species (Gribble Citation1999, Citation2000).
In this context, the traditional use of Asparagopsis (Limu kohu) in the cuisine of Hawaii, principally as a condiment, is relevant (Fortner Citation1978; Gribble Citation1999; Pukui and Elbert Citation2003). Given the range of algae and seaweeds that synthesise organo-bromine compounds (Blunt et al. Citation2006; Keng et al. Citation2013, Citation2020; Al-Adilah et al. Citation2022), and the presence of bromoform in different environments (Sturges et al. Citation1997), it is highly probably that the low-level ingestion of bromoform is more widespread than previously supposed, particularly in people who regularly consume seaweed in their diet, or seaweed-based products and supplements. It is also probable that naturally occurring bromoform may be present in herbivorous fish and enter the human food chain indirectly.
The Australia New Zealand Food Standards Code (Schedule 18) allows for chlorine and other chlorinated disinfectants to be used as processing aids for packaged water or water used as an ingredient in food, and this is a common practice globally. Low-level anthropogenic bromoform dietary exposure in humans is likely when beverages, ready to eat vegetables, meat or fish cleaning or storage processes involve use of chlorinated water to reduce aerobic microorganisms and increase product shelf life (Huang and Batterman Citation2009; Coroneo et al. Citation2017). By-products of these processes include chloroform, dibromo chloromethane, chlorodibromomethane and bromoform, although there are little published data on such sources of bromoform residues. In one study, these compounds were detected in most fish sampled with maximum values for chloroform, bromodichloromethane and bromoform at 8.3, 0.4 and 2.4 μg/kg, respectively (Delvaux et al. Citation2017). This phenomenon is likely to extend to other sectors, as noted by On et al. (Citation2008), when reviewing compounds formed during chlorinated washing of chicken meat in New Zealand. The authors noted that chloroform has been detected in chicken meat cleaned with chlorinated products and that other by-products including bromoform could be present.
Because of the various industrial applications of synthetic bromoform and the potential for human exposure from industrial or other applications, and in drinking water, its toxicology has been extensively studied and exposure guidelines have been set to protect human health. The drivers for these studies will have been the potential for significant levels of exposure, as a result of anthropogenic bromoform sources. In this context, Gribble (Citation1999) concludes that bromoform and related compounds will likely have occurred in and played a role in nature for millions of years.
Section 5: Toxicological principles and their implications
Whilst systemic exposure to significant quantities of bromoform derived from feeding livestock with Asparagopsis dry matter or Asparagopsis-derived bromoform in oil, appears to be negligible, key toxicological principles are reiterated below, as these are lacking or overlooked in a number of recent reviews. The first three principles below were noted by Glasson et al. (Citation2022), but these principles have been extended to a total of seven below. They are explicitly included to address some of the conflicting views on animal health and food quality raised by different authors and highlighted by De Bhowmick and Hayes (Citation2023) as areas of confusion. They are relevant to the early sections on residue risk and pharmacokinetics, and also the toxicology of bromoform in later sections of this paper (see Section 6). The seven principles are as below.
All things are poisonous; it is the dose that distinguishes between a therapy and a poison (Paracelsus C15th); exposure of experimental animals to toxic agents in high doses is valid to discover possible hazards in humans/animals (Casarett and Doull Citation2013; Timbrell and Barile Citation2023). Hence, stating that any compound elicits toxicity, and is a cause for concern, without providing context is meaningless.
Acute toxicity studies are used to define the doses that animals will tolerate in longer term studies (Brown Citation1980), and chronic toxicity studies are used to identify potential targets for toxicity and define lowest observed adverse effect levels (LOAELs), no observed adverse effect levels (NOAELs), Minimal Risk Levels and safety margins. Hence, identifying a level of toxicity for any compound tested in this way is inevitable.
Understanding mechanisms of toxicity and the absorption, distribution, metabolism, and excretion (ADME) of compounds is important from a toxicological perspective (Eason et al. Citation1990; Eason and O’Halloran Citation2002); pharmacokinetic studies shed light on residue concerns and risk of transfer through the food chain (Eason et al. Citation2017), as noted earlier.
The vehicle for delivery of an active ingredient has the potential to impact its bioavailability (Brown Citation1980; Rozman and Hanninen Citation1986) and therefore is an important part of an assessment. This will be important when considering future formulations and vehicles containing bromoform.
Whether or not a toxic response occurs is dependent on the properties of a compound and the exposure situation (Casarett and Doull Citation2013).
The fraction of the dose that reaches the systemic circulation (i.e. systemic bioavailability) is a key determinant of the toxic potential of a substance following oral administration (Rozman and Hanninen Citation1986).
Adverse or toxic effects are not produced by a chemical agent unless the agent reaches sites in the body at concentrations and for a period of time sufficient to produce a toxic effect (Casarett and Doull Citation2013); i.e. absorption into the blood from the gut and distribution to target organs is a prerequisite.
The implications of these principles are considered below. As noted previously, expected levels of dietary bromoform in ruminants will likely be 0.4–1.0 mg bromoform/kg liveweight, which is in the range of the reported studies in cattle (up to 0.86 mg bromoform/kg liveweight as per ). This is a low dose when compared to the doses used in toxicity studies (See Section 6). Even if 0.4–1.0 mg/kg of bromoform was 100% bioavailable (fully absorbed systemically), extrapolation from laboratory animal studies as described below, indicates adverse effects would not be expected to occur at such dose levels.
In laboratory animal toxicology studies, maximising bioavailability is desired, and this is often achieved by administration of high doses of the active ingredient (on a mg/kg basis where mg refers to the test compound and kg to the bodyweight of the animal) in solution to the test species by oral gavage (force feeding in a liquid bolus) (Casarett and Doull Citation2013), rather than ingestion at low concentrations in feed. Regardless, these animal toxicology studies are useful as they allow LOELs, NOELs, Minimal Risk Levels and safety margins to be defined for situations when bromoform is absorbed (See Section 6). Such laboratory studies in rodents also help focus health monitoring of livestock, so that appropriate blood clinical chemistry screening is undertaken (see Stefenoni et al. Citation2021). Animal toxicity studies and historical clinical experience with bromoform enable the toxic potential of bromoform to be predicted if misused. Importantly, when considering the relevance of toxicity in laboratory animals dosed with bromoform versus health effects in ruminant livestock, Kinley et al. (Citation2020) noted that no residues of bromoform were present systemically, indicating no significant absorption and no adverse health effects when A. taxiformis, containing 6.6 mg bromoform/gram was included at a rate of 0.2% in feed.
Similarly, when other studies have included Asparagopsis as a dry product or as an oil-based formulation containing Asparagopsis-derived bromoform (Li et al. Citation2018; Roque et al. Citation2019; Roque et al. Citation2021; Stefenoni et al. Citation2021; Alvarez-Hess et al. Citation2023), in appropriate proportions, most have failed to detect residues of bromoform above background concentrations found in drinking water or milk and where reported, the health of livestock has not been compromised. For example, Roque et al. Citation2019 reported good health was maintained when feeding trials extended from 14 days (Roque et al. Citation2019) to 147 days (Roque et al. Citation2021). Stefenoni et al. (Citation2021) detected very low concentrations of bromoform in the milk of a control group of dairy cows that were not fed Asparagopsis and found bromoform in the milk of cows fed a diet supplemented with Asparagopsis but the concentrations were not different from the control group.
As novel systems for the delivery of the bromoform are developed whether as Asparagopsis-based preparations (Magnusson et al. Citation2020; Alvarez-Hess et al. Citation2023), or as slow-release formulations (Ungerfeld Citation2022), it will be important to ensure that they do not lead to measurable bromoform absorption, or absorption of other halogenated compounds such as dibromomethane, over and above background or acceptable levels. Balancing safety and efficacy, new delivery formats must not reduce the effectiveness of bromoform preparations in terms of reduction in methane emissions. Alternatively, continued experimentation with different formats may lead to further improvements in safety and efficacy and ensure no risk to animal well-being.
Section 6: Toxicology at high doses in non-ruminant animals
Background
Toxicological assessments of bromoform have been undertaken following poisoning incidents in humans, and formal studies performed on rodents, using doses many orders of magnitude higher than those required to inhibit methane production in ruminants. As previously described, when Asparagopsis is included at low levels in feed formulations, a corresponding low concentration of bromoform is released into the rumen where it efficiently inhibits methanogenesis. This scenario differs from animal toxicology studies where maximising bioavailability is achieved by the administration of large doses of the active ingredient in solution to the test species by oral gavage (stomach tubes), rather than by ingestion at low concentrations in feed. Regardless, these animal toxicology studies put the risk of toxicity from bromoform into perspective when different ruminants are fed formulations with minimum effective inclusion levels of bromoform per Asparagopsis. Whilst the principles of toxicology (outlined above) suggest the relevance of this information to livestock or humans following consumption of Asparagopsis is questionable, this section of the report is included for completeness.
Toxicity is expressed in terms of their LD (50) (Dose lethal to 50% of the test population), LOAEL (Lowest observable adverse effects level), NOAEL (No observable adverse effects level).
Acute toxicity
Animal studies, combined with limited observations in humans indicate that the principal adverse health effects associated with short-term oral exposure to high levels of bromoform are CNS depression, sedation, narcosis, and sleep, and liver and kidney injury (ATSDR Citation1990; US Dept Health and Human Services Citation1993; Risher et al. Citation2005; US EPA Citation2017). The principal clinical signs in fatal cases were severe central nervous system depression with death from respiratory failure. If death could be avoided, recovery was generally complete within a few days. In the early 1900s, several deaths occurred when bromoform was given as a sedative to children (von Oettingen Citation1955). Doses were not quantified; however, it has been estimated that 250–500 mg/kg would be fatal for a 10–20 kg child (von Oettingen Citation1955). Acute toxicity tests have defined the LD50 for bromoform for female and male rats as 1,147 and 1,388 mg/kg, respectively (Chu et al. Citation1980) and 1,400 and 1,550 mg/kg for male and female mice, respectively (Bowman et al. Citation1978), indicative of low acute toxicity. To put these LD50 values of > 1,000 mg/kg into perspective, they contrast to other natural compounds that have inspired therapies and pesticides and are more toxic and have LD50 values of 50 mg/kg or less; this includes cholecalciferol (Vitamin D3) with an LD50 value of 40 mg/kg in rats, which is used therapeutically and also as a rodenticide (Eason Citation2018). More broadly, it is important to note that gut microbiota are known to have an influence on the bioavailability of many compounds (Loh et al. Citation2020; Zhang et al. Citation2021) and whilst this is likely to be the case for bromoform in livestock, additional research would be useful to gain a better understanding of the role of gut microbiota in the biotransformation of bromoform.
Chronic toxicity and target organs
There are numerous multidose toxicity studies that define target organ toxicity for bromoform. For example, when rats and mice were treated five times weekly for 13 weeks with bromoform at doses up to 400 mg/kg/day, dose-related abnormalities in liver cells were observed in males of both species (NTP, National Toxicology Program Citation1989). Condie et al. (Citation1983) administered bromoform to mice orally over the range of 72–289 mg/kg/day for 14 days and induced an elevation in serum glutamate-pyruvate transaminase (SGPT) indicative of liver damage and found related histopathology changes in animals receiving the highest dose. Munson et al. (Citation1982) administered bromoform to male and female mice by gavage at doses of 50, 125, or 250 mg/kg/day for 14 days and also detected evidence of liver and kidney damage. Liver weight and the level of a blood biomarker (serum glutamate oxaloacetate transaminase, SGOT) were increased, and serum glucose and blood urea nitrogen (BUN) levels were decreased in the high-dose animals.
Chu et al. (Citation1982) reported that administration of 5, 50, 500, or 2,500 ppm bromoform in drinking water for 90 days caused no adverse effects in male and female Sprague–Dawley rats at 5 or 50 ppm. Exposure to 500 or 2,500 ppm produced mild histological changes in the liver and thyroid that were not evident 90 days after cessation of exposure. The administration in drinking water would be a better surrogate of bromoform in feed versus the oral bolus administration used in most animal studies of this type.
Importantly in cattle, Stefenoni et al. (Citation2021) monitored health and included routine clinical chemistry on blood plasma samples. In contrast to the rodent studies (Munson et al. Citation1982; Condie et al. Citation1983), no changes were detected in blood biomarkers providing additional reassurance that key target organs such as the liver or kidneys are unaffected by feeding Asparagopsis containing bromoform.
Reproductive toxicity/Teratogenicity
Ruddick et al. (Citation1983) administered 0, 50, 100, or 200 mg/kg/day of bromoform by gavage to rats on days 6–15 of gestation. An increased incidence of minor skeletal abnormalities (appearance of a 14th rib, intraparietal deviations, and delayed ossification of sternebrae), was seen in developing foetuses at 100 and 200 mg/kg/day.
Genetic toxicity and carcinogenicity
Some epidemiological studies suggest that there may be an association between exposure to trihalomethanes in drinking water and increased frequencies of cancers of the stomach, colon, rectum, or pancreas and there are somewhat conflicting conclusions from studies exploring associations between trihalomethanes with bladder cancer (Evlampidou et al. Citation2020; Helte et al. Citation2022). However, these studies do not provide information as to whether the observed effects are due to bromoform or to one or more of the numerous other by-products also present in chlorinated water, compounded in some cases by the variability in the historical data collated on trihalomethane concentrations in drinking water (Evlampidou et al. Citation2020). Formally, evidence for carcinogenicity of bromoform in humans is considered inadequate to make an assessment (USEPA Citation1989).
The results from 42 short-term genetic tests are summarised in a short review (IARC Citation1991). As with other test systems, high doses will have been used in these studies. Whilst most studies yielded negative outcomes the results were inconsistent, with some positive effects. Unscheduled DNA synthesis was not induced in hepatocytes and binding to DNA was not observed in the liver and kidney of rats treated in vivo. The overall evaluation by the IARC concluded that there is limited evidence in experimental animals for carcinogenicity associated with bromoform.
It is apparent that the supplier of chemical reagents, Sigma-Aldrich Merck has addressed the inconsistencies in historical testing dating back to the 1970's given new information now presented in their 2024 Safety Data Sheet for bromoform (Sigma-Aldrich Citation2024). They cite the results of six genetic toxicity tests from studies conducted to current OECD guidelines likely to be as part of in-house processes which include toxicology assessment of products listed in their catalogue. Unfortunately, these reports are not published but the safety data sheet information is informative: five out of six of these studies provided “negative” outcomes indicating a lack of genotoxicity.Footnote1 In an early response to concern regarding drinking water safety, bromoform had been assessed and compared with other disinfection by-products for their mutagenic potency. The order of the mutagenic potency was 3-chloro-4-(dichloromethyl)-5-hydroxy-2[5H]-furanone > bromoacetic acid > dibromoacetic acid > dichloroacetic acid > chloroacetic acid; tribromoacetic acid, trichloroacetic acid, bromoform, and chloroform CF were assessed as not mutagenic (Kargalioglu et al. Citation2002). These results align with the recently summarised results in the Sigma-Aldrich Safety Data Sheet on bromoform (Sigma-Aldrich Citation2024).
Carcinogenicity is assessed by lifetime studies in rodents. Rats and mice have been orally administered bromoform for 5 days/week for 2 years, at doses of 0, 100, or 200 mg/kg/day (NTP Citation1989). No evidence was observed of carcinogenic activity in mice. Neoplastic lesions (adenomatous polyps or adenocarcinomas) were observed in the colon or rectum of 3/50 male rats treated with 200 mg/kg/day and in 1/50 or 8/50 female rats treated with 100 or 200 mg/kg/day, respectively. Based on the occurrence of these neoplasms of the large intestine, the NTP (Citation1989) concluded that there is some evidence of carcinogenic activity for male rats and clear evidence of carcinogenic activity for female rats. However, in a 24-month dietary study (Faust Citation1995), there was no evidence of carcinogenic activity in male or female rats administered microencapsulated bromoform at concentrations of 400, 1600, or 6500 ppm (20, 80, or 325 mg/kg). Importantly there are no reports of bladder abnormalities in these high dose animal studies with bromoform administered as a sole agent.
Regardless of the presence or absence of toxic effects in these and other studies, the amounts of bromoform being assessed are not relevant to amounts delivered by dietary administration of bromoform per Asparagopsis or other low level release systems to ruminant livestock.
Extrapolation from animal studies
This section illustrates how animal toxicology study data are used by WHO and other bodies such as the US Agency for Toxic Substances and Disease Registry (ATSDR) when undertaking toxicological profiles and providing advice on health effects. Minimal Risk Levels are estimates of daily human exposure that are likely to be without adverse health effects. Derivation of three types of Minimal Risk Levels is described below.
An acute-duration oral Minimal Risk Level of 0.7 mg/kg/day has been derived for bromoform. This Minimal Risk Level is based on a NOAEL of 72 mg/kg/day and a LOAEL of 145 mg/kg/day for centrilobular pallor in mice receiving gavage doses of bromoform for 14 days (Condie et al. Citation1983). The Minimal Risk Level was derived by dividing the NOAEL by an uncertainty factor of 100 (10 for animal to human extrapolation and 10 for human variability).
An intermediate-duration oral Minimal Risk Level of 0.2 mg/kg/day has been derived for bromoform. This is based on a NOAEL of 50 mg/kg for hepatocellular vacuolisation in rats administered gavage doses of bromoform in corn oil 5 days/week for 13 weeks (NTP Citation1989). The Minimal Risk Level was derived by dividing the duration adjusted NOAEL of 18 mg/kg/day by an uncertainty factor of 100 (10 for animal to human extrapolation and 10 for human variability).
A chronic-duration oral Minimal Risk Level of 0.02 mg/kg/day has been derived for bromoform. This Minimal Risk Level is based on a LOAEL of 100 mg/kg for hepatocellular vacuolisation in rats administered gavage doses of bromoform in corn oil 5 days/week for 2 years (NTP Citation1989). The Minimal Risk Level was derived by dividing the duration adjusted LOAEL of 71 mg/kg/day by an uncertainty factor of 300 (3 for use of a minimal LOAEL, 10 for animal to human extrapolation, and 10 for human variability) and a modifying factor of 10 to account for the identification of a lower LOAEL in a 13-week study (NTP Citation1989).
A number of these Minimal Risk Level values, albeit set for humans, are lower than the doses that ruminant livestock will be exposed to, namely 0.4–1.0 mg/kg/day. Importantly the human Minimal Risk Levels values are generated from studies where dosing regimens are designed to deliver considerable absorption and distribution of bromoform throughout the body, with the intent being assessment of target organ toxicity. This is in marked contrast to the delivery of bromoform to ruminants where the reticulo-rumen is the target and to address safety concerns, doses and delivery systems are designed to ensure minimal or no absorption.
Relevance
It is suggested by some authors (Abbott et al. Citation2020; Roskam et al. Citation2022) that, as Asparagopsis contains bromoform and that it is toxic, Asparagopsis should be avoided for methane suppression (Abbott et al. Citation2020; Roskam et al. Citation2022); De Bhowmick and Hayes (Citation2023) note there are conflicting views. In response to these conflicting views, toxicological principles underpinning safety assessment have been presented alongside a comprehensive review of bromoform animal toxicology and pharmacokinetics.
The safety assessment in this review has focused on bromoform for a number of reasons:
Bromoform is the most abundant of the bioactive organobromines in Asparagopsis by an order of magnitude over the next most abundant compound, dibromochloromethane (Glasson et al. Citation2022).
There is potential for slow-release formulations, which will only contain bromoform, building on the research described above with bromoform delivered in an oil.
Delivery of small amounts of bromoform is being targeted to ensure that it does not lead to measurable bromoform absorption. In this scenario bromoform metabolites should not be a safety concern.
By contrast, exposure to high levels of bromoform is clearly hazardous for humans and laboratory animals. It is important to note that adverse effects are to be expected in animal studies of the type described above, given their design and purpose. However, they do not preclude the proper use of natural or synthetic substances in human or veterinary applications, even when absorption is needed for efficacy. This is the case for natural compounds and synthetic substances when the mode and site of action, pharmacokinetics and mechanism of toxicity are understood.
While bromoform intake limits are yet to be defined for cattle specifically, the US EPA (Citation2017) has suggested a reference dose for bromoform, an estimated level of daily oral exposure without negative effects, to be 0.02 mg/kg/day for human consumption; similarly, the WHOFootnote2 has a Tolerable Daily Intake (TDI) of 17.9 µg/kg (0.018 mg/kg/day) of body weight.
The International Agency for Research on Cancer has placed bromoform in Group 3, which means not classifiable as carcinogenic to humans.Footnote3 The World Health Organization (WHO) has set up a guideline value of 100 µg/l (0.1 mg/l), of bromoform as a disinfection by-product in drinking water, and a TDI of 17.9 µg/kg of body weight as above. The WHO guidelines for drinking water align with the Maximum Acceptable Values in drinking water standards for New Zealand of 0.1 mg/l and in the US at 80 µg/l (0.08 mg/litre).
The guideline value in water of 100 µg/l compares favourably with the background level of bromoform recorded in treated drinking water which typically can range from < 1–10 µg/l, with higher values in some instances (IARC Citation1991), and also the background recorded in the milk of cows not fed Asparagopsis of 16.5 µg/l reported by Stefenoni et al. (Citation2021) and the highest value seen in milk of 35 µg/l in the Muizelaar et al. (Citation2021) study. The Tolerable Daily Intake (TDI) for bromoform of 18 µg/kg body weight would require an intake of 1.25 mg in a 70 kg person, equivalent to 35 litres of milk daily at the highest value seen in milk of 35 µg/l.
Section 7: Other exposure and regulatory considerations
New Zealand Maximum Residue Levels (MRLs) are established under the Food Act 2014 Section 383(8)(a) that empowers the chief executive of New Zealand’s Ministry for Primary Industries (MPI) to specify the maximum amounts of contaminants or residues that may be present in food. Regulatory requirements for use of methane inhibitors are being developed in New Zealand. Present New Zealand regulatory direction is that inhibitors fed to animals are to be considered as veterinary medicines, which would necessitate establishment of a Maximum Residue Level (MRL) value. When setting or amending MRLs, the Director General must take into account the following.
the need to protect public health.
the desirability of avoiding unnecessary restrictions on trade.
the desirability of maintaining consistency between New Zealand’s food standards and those standards that apply internationally.
New Zealand’s obligations under any relevant international treaty, agreement, convention, or protocol.
Regulatory requirements for use of feed-applied inhibitors are under development in New Zealand, as are considerations of a Maximum Residue Level (MRL) value. In this respect, the key factor to consider is that it can be expected that a variable baseline/background level of bromoform in milk, and other food products likely exists. This is due to the expected background levels of bromoform in the environment, including drinking water and other natural and anthropogenic sources which may also enter the livestock production system. Residues of bromoform may be detectable, albeit at low levels, in milk and other tissues of the body. However, given this variable baseline which likely masks any potential residues derived from Asparagopsis or from any other delivery format, it could be argued that there is limited ability and value to be had in establishing a MRL value, linked to the use of bromoform alone. Occurrence of bromoform in milk from untreated dairy cattle in some studies indicates there are additional dietary sources. An approach to exempt bromoform from a MRL could be feasible as the variable baseline in milk indicates a limited ability to regulate good agricultural practice based on bromoform residues, when sources include drinking water, and other foods. The rationale behind a MRL exemption, which includes human exposure at low levels from a variety of sources, would also remain valid for assessing impacts on international trade. However, it is noted that international Codex Alimentarius considerations may require a MRL.
An alternative approach would be to adopt an accepted (e.g. New Zealand) drinking water Maximum Acceptable Value (MAV) of 100 µg/l (0.1 mg/L) This aligns with the WHO recommended guideline (MoH Citation2016; WHO Citation2022), as a MRL for all beverages and food. This would, in effect, provide a MRL in beverages, including milk of 100 µg/l (0.1 mg/L) and in food stuffs 100 µg/kg (0.1 mg/kg). While the impacts on public health from an exemption approach from a MRL are assessed as being low, an aligned MAV and MRL of 100 µg/l (0.1 mg/L) in all beverages, and in all foods of 100 µg/kg (0.1 mg/kg) would provide additional reassurances. In particular, it would align with the drinking water standard, and capture all dietary sources of bromoform, versus targeting just one potential source.
Aligned with this proposal, an estimate of dietary exposure has been undertaken to assess whether the potential range of occurrence in milk and meat might present a public health concern. The model diet used in veterinary medicine dietary exposure estimates (by the Joint WHO/FAO Expert committee on Food Additives (JECFA)) has been used to inform intakes (WHO Citation2012). A worst-case baseline exposure based on background presence of bromoform in drinking water up to the MAV () has been undertaken to understand the potential elevation that may result from use of bromoform as a methane inhibitor. Values have been compared against the WHO Tolerable Daily Intake of 0.018 mg/kg bodyweight/day, as this health-based guidance value is currently referred to in the New Zealand drinking water standards.
Table 2. Estimated dietary exposure from bromoform for a 70 kg adult based on reported background values in milk from untreated animals and with allowance for drinking water standards.
The elevation in dietary exposure as a consequence of bromoform entering the food supply, over the worst case for baseline exposure in milk and water is only 2% of the WHO TDI, indicating a minimal risk to public health. Internationally, these factors may be impacting new regulatory considerations. For example, the California Department of Food and Agriculture (CDFA) Livestock Drug Program issued a ‘letter of no objection’ for the Asparagopsis taxiformis sale as a digestive aid product (De Bhowmick and Hayes Citation2023) acknowledging the levels of bromoform and iodine in this product are in accordance with what is acceptable by the US Environmental Protection Agency (EPA 2022). Commercial sale and supply of Asparagopsis species to cattle producers began in 2022 via CH4Global (De Bhowmick and Hayes Citation2023) in the US.
Conclusions
Asparagopsis as a dietary intervention for enteric methane production shows considerable promise in reducing enteric methane production by livestock. Naturally occurring bromoform in Asparagopsis is considered responsible for this effect and re-analysis of recent studies, with seaweed or an oil-based delivery vehicle as part of the diet, shows a dose–response between the effect on methane emissions and bromoform ingested (in mg/kg/day) in sheep and cattle.
For most compounds, to produce systemic pharmacological or toxicological effects, absorption into the blood from the gut and distribution to target organs is a prerequisite. Importantly, the evidence presented here shows that this is not the case for bromoform released from Asparagopsis delivered to ruminants in feed or when delivered in oil at low concentrations. The target site is within the rumen and absorption of bromoform is neither required nor is it desirable. Bromoform, delivered in these ways, is not unique in its ability to have its desired effect with no or very limited systemic absorption. There are drugs which are not absorbed, such as cholestyramine (a hypolipidemic agent), which act by sequestering bile acids in the gut (Malloy and Kane Citation2018).
There is no evidence of risk to animals and specifically to their health when Asparagopsis in feed is part of a rational feeding regimen or when Asparagopsis or bromoform (without Asparagopsis biomass) are delivered in oil. The risk to consumers from the transfer of bromoform from Asparagopsis dry matter or oil- based formats in the ruminant diet to edible parts of the animal (carcass/ muscle or organs such as liver, kidney or milk products) is very low to negligible. The occurrence of bromoform in some samples of control milk from untreated cattle indicates there are additional dietary or environmental sources. There is a large range of natural and anthropogenic sources of exposure to bromoform such as chlorinated disinfectants and drinking water, and any potential additive effect derived from bromoform in an appropriate delivery vehicle appears to be negligible.
In response to conflicting views in the literature regarding safety, animal health and food quality, we have more fully explored the toxicology and metabolism of bromoform. Health benefits or toxicity associated with ingestion of any material will depend largely on how it is administered. The amounts of material taken into the body and the duration of exposure will be critical to the presence or absence of toxic effects. Bromoform is no exception and oral administration over long periods in monogastric animals has been associated with a range of toxic effects. For adverse effects, such as liver damage, or carcinogenic effects to occur, absorption of significant amounts of bromoform into the blood stream and distribution through the body, followed by sustained exposure is necessary. These systemic toxic effects in laboratory animals, in both acute and long-term toxicology studies, are relevant only to the assessment of adverse health risk in livestock if bromoform is absorbed, and concentrations in the body exceed the low levels that are likely to be present from a range of natural and anthropogenic sources. This will apply to bromoform whether it be naturally occurring (such as that present in seaweed or in recently developed oil-based formulations) or synthetic (when delivered as part of the diet) if the bromoform is retained in the reticulo-rumen. If bromoform is confined to the foregut (reticulo-rumen) and degraded/captured in the foregut of ruminants and no or minimal absorption occurs, then laboratory toxicology animal studies, and the associated safety margins and toxicity classification defined by these studies should be noted but are of limited relevance to the health of ruminant livestock and consumers. Similar considerations would apply to bromoform delivered from a slow-release intra-ruminal bolus where bromoform is released, over a period of weeks to months, at rates similar to those achieved to date by appropriate delivery in seaweed or an oil-based vehicle as part of the diet. In terms of regulatory requirements for use of feed applied inhibitors and considerations of a Maximum Residue Level (MRL) value for bromoform, we conclude that an appropriate approach would be to adopt the New Zealand drinking water Maximum Acceptable Value (MAV) of 100 µg/l (0.1 mg/L). This aligns with the WHO recommended guideline (MoH Citation2016; WHO Citation2022), as a MRL for all beverages and food. This would, in effect, provide a MRL in beverages, including milk of 100 µg/l (0.1 mg/L) and in food stuffs of 100 µg/kg (0.1 mg/kg).
Looking to the future, several commercial and technical risk mitigation strategies are suggested to build on the existing safety and efficacy database for Asparagopsis and bromoform when used for reducing methane emissions. Recommendations include consideration of the implications of novel formats or storage vehicles; it would also be of interest to have a better understanding of the fate of bromoform in the reticulo-rumen. Further measurement of background sources of bromoform residues in drinking water, and other beverages and foods, including vegetables, meat or fish where cleaning or storage processes involve the use of chlorinated water, should ensure that concentrations, from this variety of sources, are within the MRL proposed above. In addition, screening for the presence of naturally-occurring bromoform in other fish and seaweed products would shed light on the variety of routes by which naturally occurring or anthropogenic bromoform might enter the food chain. Comparison of these results (when rates of 1.0 mg/kg/day or less of bromoform are delivered) versus any potential additive effect derived from delivery of bromoform, which at this time appears to be negligible, would be pertinent. Further specific recommendations are below.
It is to be expected that novel systems for storage/preservation of the bioactive bromoform will be developed to improve stability and extend the shelf-life of delivery format. These may involve alternatives to freeze drying of Asparagopsis such as oils, or slow-release formulations of Asparagopsis or of synthetic bromoform (Magnusson et al. Citation2020; Ungerfeld Citation2022; Alvarez-Hess et al. Citation2023). In this context, suggestions that products should be tailored to maximise the bioactivity (bromoform content) (Wheeler et al. Citation2021) should be pursued with caution, unless it can be shown that absorption of bromoform from these novel formats does not exceed baseline levels that can be attributed to anthropogenic exposures to bromoform and negate the benefit of seaweed as a natural and innocuous delivery system. Alternatively, experimentation with different formats to further optimise versus maximise bromoform content and delivery vehicles, may lead to further improvement in safety and efficacy and animal well-being.
It will be important to continue to monitor the concentrations of bromoform and other halogenated compounds of interest, including metabolites and those present in Asparagopsis or other vehicles for delivery of bromoform to the reticulo-rumen. Bromoform should be delivered at the appropriate amounts, to achieve the desired effects on methane emissions, while minimising the amount and duration of Asparagopsis and/or bromoform intake to achieve these targets. It will also be important to conduct further analyses of blood, milk, meat, and other tissues to assess the evidence for any systemic absorption of bromoform, or the presence of bromoform metabolites. This could include monitoring future trials with more formal assessment of systemic bioavailability. Formal bioavailability studies typically look at the fraction of the dose that is bioavailable by comparison of the blood or plasma concentrations over time and calculate area under the curve (AUC) following oral administration of the bromoform in solution versus a standard dietary route (in the desired vehicle). In this case, an AUC of zero for bromoform in plasma over time would be the desired outcome following provision of bromoform to a ruminant and this would contrast with a quantifiable AUC from administration of bromoform in solution at the same dose, on a mg/kg basis (0.4–1.0 mg/kg) as above. Complementary studies could confirm metabolism by rumen microbiota, and subsequent excretion of either unchanged bromoform or metabolites.
There is a case to extend health monitoring in trials to include further routine clinical chemistry on blood plasma/serum samples, alongside residue analyses, as well as post-mortem pathology to assess key target organs such as the liver. Future residue monitoring studies must include control groups and the drinking water and other feed should be monitored for bromoform during the trials. Care should also be taken if urine is to be sampled that it is not inadvertently contaminated with faeces as there would be a possibility of false positive results in urine which would imply greater absorption of bromoform than is the case.
Asparagopsis is a potential source of iodine. The presence of iodine in milk is currently captured in the Food Notice: Maximum Residue Levels for an Agricultural Compound under an MRL exemption as a topical bactericide in food producing animals, which encompasses teat sanitisers in dairy animals. Nevertheless, it will be important to minimise the amount of additional iodide load in the ruminant diete (Lean et al. Citation2021). With this in mind, it will be wise to ensure that dried Asparagopsis or and novel oil-based or formulations as feed additives contain high enough concentrations of bromoform, such that desired amounts of bromoform are ingested with a smaller amount of seaweed, and consequently less total iodide in the diet. If oil-based or alternative slow-release bromoform delivery systems partially replace the need for Asparagopsis, then concerns regarding iodine will be avoided.
Acknowledgment
We acknowledge Methane Mitigation Ventures Ltd for commissioning this review in December 2021 including its significant financial support in the preparation of this paper and supporting background information. Acknowledgement is also made to New Zealand's Ministry for Primary Industries for financial support and background information via its Sustainable Food and Fibre Futures (SFFF21001, Preliminary evaluation of potential feed-based commercial solutions to the problem of ruminant methane emissions). Andrew Pearson from Tonkin and Taylor is also acknowledged for his contribution to an early draft of this review. We thank Dr Neville Jopson for statistical advice relating to the analysis of the published trial data.
Disclosure statement
As indicated above funding support was provided by Methane Mitigation Ventures.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/00288233.2024.2355674)
Notes
1 Test Type: Ames test: S. typhimurium Metabolic activation: with and without metabolic activation Method: OECD Test Guideline 471 Result: positive.
Test Type: sister chromatid exchange assay test system: Chinese hamster ovary cells Metabolic activation: Metabolic activation Method: OECD Test Guideline 479 Result: negative.
Test Type: In vitro mammalian cell gene mutation test system: mouse lymphoma cells Metabolic activation: Metabolic activation Method: OECD Test Guideline 490 Result: negative.
Test Type: Chromosome aberration test in vitro test system: Chinese hamster ovary cells Metabolic activation: with and without metabolic activation Method: OECD Test Guideline 473 Result: negative.
Test Type: unscheduled DNA synthesis assay Species: Rat Application Route: Oral Method: OECD Test Guideline 486 Result: negative.
Test Type: In vivo micronucleus test Species: Mouse Application Route: Oral Method: OECD Test Guideline 474 Result: negative.
2 Extract from WHO Guidelines for drinking-water quality. 2017, https://www.who.int/water_sanitation_health/dwq/2edvol1c.pdf. Bromoform is readily absorbed from the gastrointestinal tract. In experimental animals, long-term exposure to high doses causes damage to the liver and kidney. In one bioassay, bromoform induced a small increase in relatively rare tumours of the large intestine in rats of both sexes but did not induce tumours in mice. Data from a variety of assays on the genotoxicity of bromoform are equivocal. IARC has classified bromoform in Group 3. A TDI was derived on the basis of a NOAEL of 25 mg/kg of body weight per day for the absence of histopathological lesions in the liver in a well-conducted and well-documented 90-day study in rats. This NOAEL is supported by the results of two long-term studies. The TDI is 17.9 µg/kg of body weight, correcting for exposure on 5 days per week and using an uncertainty factor of 1000 (100 for intra – and interspecies variation and 10 for possible carcinogenicity and the short duration of the study). With an allocation of 20% of the TDI to drinking water, the guideline value is 100 µg/litre (rounded figure). This aligns with NZ and US guidelines for drinking water.
References
- Abbott W, Aasen IA, Beauchemin KA, Grondahl F, Gruninger R, Hayes M, Huws S, Kenny DA, Krizsan SJ, Kirwan SF, et al. 2020. Seaweed and seaweed bioactives for mitigation of enteric methane: challenges and opportunities. Animals. 10(12):2432. doi:10.3390/ani10122432.
- Agency for Toxic Substances and Disease Registry (ATSDR). 1990. Toxicological Profile for Bromoform and Chlorodibromomethane. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA.
- Ahmed AE, Kubic VL, Anders MW. 1977. Metabolism of haloforms to carbon monoxide. I. In vitro studies. Drug Metab Dispos. 5:198–204.
- Al-Adilah H, Feiters MC, Carpenter LC, Kumari P, Carrano CJ, Al-Bader D, Kuepper F. 2022. Halogens in seaweeds: biological and environmental significance. Phycology. 2:132–171. doi:10.3390/phycology2010009.
- Alvarez-Hess PS, Jacobs JL, Kinley RD, Roque BM, Neachtain AS, Chandra S, Williams SR. 2023. Twice daily feeding of canola oil steeped with Asparagopsis armata reduced methane emissions of lactating dairy cows. Anim Feed Sci Technol. 297:115579. doi:10.1016/j.anifeedsci.2023.115579.
- Andreen DM, Billman ED, Brito AF, Soder KJ. 2023. Effect of incremental amounts of Asparagopsis taxiformis on ruminal fermentation and methane production in continuous culture with orchardgrass herbage. Anim Feed Sci Technol. doi:10.1016/j.anifeedsci.2023.115641.
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. 2006. Marine natural products. Nat Prod Rep. 23:26–78. doi:10.1039/b502792f.
- Bowman FJ, Borcelleca JF, Munson AE. 1978. The toxicity of some halomethanes in mice. Toxicol Appl Pharmacol. 44:213–215. doi:10.1016/0041-008X(78)90300-9.
- Brown VK. 1980. Acute toxicity in theory and practice. Chichester/New York, NY: Wiley; p. 159.
- Casarett LJ, Doull J. 2013. Casarett and Doull's toxicology: the basic science of poisons. Klaassen CD, editor. 8th ed. New York (NY): McGraw-Hill Education. ISBN 978-0-07-176923-5.
- Chu I, Secours V, Marino I, Villeneuve DC. 1980. The acute toxicity of four trihalomethanes in male and female rats. Toxicol Appl Pharmacol. 52(2):351–353. doi:10.1016/0041-008X(80)90122-2.
- Chu I, Villeneuve DC, Secours VE, Becking GC, Valli VE. 1982. Trihalomethanes: II reversibility of toxicological changes produced by chloroform, bromodichloromethane, chlorodibromomethane and bromoform in rats. J Environ Sci Health Part B. 17(3):225–240. doi:10.1080/03601238209372315.
- Class T, Kohnle R, Ballschmiter K. 1986. Chemistry of organic traces in air: bromo- and bromochloroethanes in air over the Atlantic Ocean. Chemosphere. 15:429–436. doi:10.1016/0045-6535(86)90536-9.
- Condie LW, Smallwood CL, Laurie RD. 1983. Comparative renal and hepatotoxicity of halomethanes: bromodichloromethane, bromoform, chloroform, dibromochloromethane and methylene chloride. Drug Chem Toxicol. 6(6):563–578. doi:10.3109/01480548309017810.
- Coroneo V, Coroneo V, Marras B, Marrucci A, Succa S, Meloni B, Pinna A, Angioni A, Sanna A, Schintu M. 2017. Presence of trihalomethanes in ready-to-eat vegetables disinfected with chlorine. Food Addit. Contam. Part A. 34:2111–2117. doi: 10.1080/19440049.2017.1382723
- De Bhowmick G, Hayes M. 2023. Potential of seaweeds to mitigate production of greenhouse gases during production of ruminant proteins. Global Chall. 7(5):2200145. doi: 10.1002/gch2.202200145
- Delvaux NA, de Queiroz MELR, Neves AA, Oliveira AF, da Silva MRF, Faroni LRA, Heleno FF. 2017. Headspace solid phase microextraction-gas chromatography for the determination of trihalomethanes in fish. Microchem J. 133:539–544. doi:10.1016/j.microc.2017.04.019.
- Dijkstra J, Bannink A, France J, Kebreab E, van Gastelen S. 2018. Short communication: antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J Dairy Sci. 101:9041–9047. doi:10.3168/jds.2018-14456.
- Du Plessis M, Möller I, Liebenberg L. 2020. Double rarity: an unusual case of bromoform poisoning detected by post-mortem radiography. Int J Legal Med. 134:703–708. doi:10.1007/s00414-019-02032-1.
- Eason CT. 2018. Connections between rodenticides & drugs: a review of natural compounds with ecological, biocidal & medical applications. NZ J Zool. 45(1):1–12. doi:10.1080/03014223.2017.1348956.
- Eason CT, Bonner FW, Parke DV. 1990. The importance of pharmacokinetic and receptor studies in drug safety evaluation. Regul Toxicol Pharmacol. 11(3):288–307. doi:10.1016/0273-2300(90)90028-A.
- Eason CT, O’Halloran K. 2002. Biomarkers in toxicology versus ecological risk assessment. Toxicology. 181-182:517–521. doi:10.1016/S0300-483X(02)00472-9.
- Eason CT, Shapiro L, Ogilvie S, King C, Clout M. 2017. Trends in the development of mammalian pest control technology in New Zealand. NZ J Zool. 44(4):267–304. doi:10.1080/03014223.2017.1337645.
- EC (European Council). 2020. Directive (EU) 2020/2184 of the European Parliament and of the council of 16 December 2020 on the quality of water intended for human consumption (recast). OJ L. 435:1–62.
- Evlampidou I, Font-Ribera L, Rojas-Rueda D, Gracia-Lavedan E, Costet N, Pearce N, Vineis P, Jaakkola JJK, Delloye F, Makris KC, et al. 2020. Trihalomethanes in drinking water and bladder cancer burden in the European Union. Environ Health Perspect. 128(1):17001. doi:10.1289/EHP4495.
- Faust RA. 1995. Toxicology Profiles. Formal Toxicity Summary for Bromoform. The Risk Assessment Information System. Chemical Hazard Evaluation Group, Biomedical and Environmental Information Analysis Section, Health Sciences Research Division, Oak Ridge, Tennessee. No longer available (previously https://rais.ornl.gov/tox/profiles/bromoform).
- Fennessy PF, Proctor LE, Muetzel S. in preparation. Methane mitigation in sheep – response to Asparagopsis.
- Firkins JL, Mitchell KE. 2023. Rumen modifiers in today's dairy rations. J Dairy Sci. doi:10.3168/jds.2022-22644.
- Fortner HJ. 1978. The Limu Eater: a cookbook of Hawaiian seaweed. HI: University of Hawaii Sea Grant College Program.
- Glasson CR, Kinley RD, de Nys R, King N, Adams SL, Packer MA, Svenson J, Eason CT, Magnusson M. 2022. Benefits and risks of including the bromoform containing seaweed asparagopsis in feed for the reduction of methane production from ruminants. Algal Res. 64:102673. doi:10.1016/j.algal.2022.102673.
- Goodwin KD, North WJ, Lidstrom ME. 1997. Production of bromoform and dibromomethane by Giant Kelp: factors affecting release and comparison to anthropogenic bromine sources. Limnol Oceanogr. 42(8):1725–1734. doi:10.4319/lo.1997.42.8.1725.
- Gribble GW. 1999. The diversity of naturally occurring organobromine compounds. Chem Soc Rev. 28(5):335–346. doi:10.1039/a900201d.
- Gribble GW. 2000. The natural production of organobromine compounds. Environ Sci Pollut Res Int. 7(1):37–49. doi:10.1065/espr199910.002.
- Gschwend PM, MacFarlane JK, Newman KA. 1985. Volatile halogenated organic compounds released to seawater from temperate marine macroalgae. Science. 227:1033–1035. doi:10.1126/science.227.4690.1033.
- Helte E, Säve-Söderbergh S, Ugge H, Fall K, Larsson S, Åkesson A. 2022. Chlorination by-products in drinking water and risk of bladder cancer – A population-based cohort study. Water Res. 214:118202. doi:10.1016/j.watres.2022.118202.
- Hladik ML, Hubbard LE, Kolpin DW, Focazio MJ. 2016. Dairy-impacted wastewater is a source of iodinated disinfection byproducts in the environment. Environ Sci Technol Lett. 3(5):190–193. doi:10.1021/acs.estlett.6b00109.
- Huang AT, Batterman S. 2009. Formation of trihalomethanes in foods and beverages. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 26(7):947–957. doi:10.1080/02652030902897739.
- International Agency for Research on Cancer (IARC). 1991. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 52.
- Jia Y, Quack B, Kinley RD, Pisso I, Tegtmeier S. 2022. Potential environmental impact of bromoform from asparagopsis farming in Australia. Atmos Chem Phys. 22(11):7631–7646. doi:10.5194/acp-22-7631-2022.
- Kargalioglu Y, McMillan B, Minear R, Plewa M. 2002. Analysis of the cytotoxicity and mutagenicity of drinking water disinfection by-products in Salmonella typhimurium. Teratogenesis, Carcinogenesis and Mutagenesis. 22(2):113–128. doi: 10.1002/tcm.10010
- Keng FSL, Phang SM, Rahman A, Leedham EC, Gill M, Sturges WT. 2020. The emission of volatile halocarbons by seaweeds and their response towards environmental changes. J Appl Phycol. 32(2):1377–1394. doi:10.1007/s10811-019-02026-x.
- Keng FSL, Phang SM, Rahman NA, Leedham EC, Hughes C, Robinson AD, Harris NRP, Pyle JA, Sturges WT. 2013. Volatile halocarbon emissions by three tropical brown seaweeds under different irradiances. J. Appl. Phycol. 25:1377–1386. doi:10.1007/s10811-013-9990-x.
- Kinley RD, Martinez-Fernandez G, Matthews MK, de Nys R, Magnusson M, Tomkins NW. 2020. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J Clean Prod. 259:120836. doi:10.1016/j.jclepro.2020.120836.
- Lean IJ, Golder HM, Grant TMD, Moate PJ. 2021. A meta-analysis of effects of dietary seaweed on beef and dairy cattle performance and methane yield. PLoS One. 16(7):e0249053. doi: 10.1371/journal.pone.0249053
- Li X, Norman HC, Kinley RD, Laurence M, Wilmot M, Bender H, de Nys R, Tomkins N. 2018. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim Prod Sci. 58(4):681–688. doi:10.1071/AN15883.
- Liu H, Wang J, Wang A, Chen J. 2011. Chemical inhibitors of methanogenesis and putative applications. Appl Microbiol Biotechnol. 89:1333–1340. doi:10.1007/s00253-010-3066-5.
- Loh ZH, Ouwerkerk D, Klieve AV, Hungerford NL, Fletcher MT. 2020. Toxin degradation by rumen microorganisms: a review. Toxins. 12(10):664. doi:10.3390/toxins12100664.
- Lucas GHW. 1928. A study of the fate and toxicity of bromine and chlorine containing anesthetics. J Pharmacol. 34:223–237.
- Machado L, Magnusson M, Paul NA, de Nys R, Tomkins N. 2014. Effects of marine and freshwater macroalgae on in-vitro total gas and methane production. PloS One. 9(1):e85289. doi:10.1371/journal.pone.0085289.
- Magnusson M, Vucko MJ, Neoh TL, de Nys R. 2020. Using oil immersion to deliver a naturally-derived, stable bromoform product from the red seaweed Asparagopsis taxiformis. Algal Res. 51:102065. doi:10.1016/j.algal.2020.102065.
- Malloy MJ, Kane JP. 2018. Agents used in dyslipidemia. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and clinical pharmacology. 12th ed. New York, NY: McGraw-Hill Medical; Chapter 35.
- Ministry of Health (MoH). 2016. Guidelines for drinking-water quality management for New Zealand. Wellington: MoH.
- Mink FL, Brown TJ, Rickabaugh J. 1986. Absorption, distribution, and excretion of 14C-trihalomethanes in mice and rats. Bull Environ Contam Toxicol. 37(5):752–758. doi:10.1007/BF01607835.
- Muizelaar W, Groot M, van Duinkerken G, Peters R, Dijkstra J. 2021. Safety and transfer study: transfer of bromoform present in Asparagopsis taxiformis to milk and urine of lactating dairy cows. Foods. 10(3):584. doi:10.3390/foods10030584.
- Muñoz-Tamayo R, Chagas JC, Ramin M, Krizsan SJ. 2021. Modelling the impact of the macroalgae Asparagopsis taxiformis on rumen microbial fermentation and methane production. Peer Community J. 1: article (e7). doi:10.24072/pcjournal.11.
- Munson AE, Sain LE, Sanders VM, et al. 1982. Toxicology of organic drinking water contaminants: trihalomethane, bromodichloromethane, dibromochloromethane and tribromomethane. Environ. Health Perspect. 46:117–126. doi:10.1289/ehp.8246117.
- Nelson WA, Neill K, D’Archino R, Rolfe JR. 2019. Conservation status of New Zealand macroalgae. Dept of Conservation. New Zealand Threat Classification Series 30.
- Nokes CJ. 1999. Disinfection by-products in New Zealand drinking waters: occurrence, controlling factors and management. ESR Client Report FW9978.
- NTP (National Toxicology Program). 1989. Toxicology and Carcinogenesis Studies of Tribromomethane (Bromoform) (CAS No. 75-25-2) in F344/N Rats and B6C3F1 Mice (Gavage Studies). U.S. Department of Health and Human Services. NTP Technical Report Series No. 350. https://ntp.niehs.nih.gov/.
- On S, Cressey P, Vannort R. 2008. Compounds formed during chlorinated wash of chicken meat. ESR Client Report FW0833.
- Parra P, Martinez E, Sunol C, Artigas F, Tusell JM, Gelpl E, Albaiges J. 1986. Analysis, accumulation and central effects of trihalomethanes. I. Bromoform. Toxicol Environ Chem. 11:79–91. doi:10.1080/02772248609357122.
- Patra A, Park T, Kim M, Yu Z. 2017. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J Anim Sci Biotechnol. 8:13. doi:10.1186/s40104-017-0145-9.
- Paul NA, Cole L, de Nys R, Steinberg PD. 2006. Ultrastructure of the gland cells of the red alga Asparagopsis armata (Bonnemaisoniaceae). J Phycol. 42:637–645. doi:10.1111/j.1529-8817.2006.00226.x.
- Pukui MK, Elbert SH. 2003. “Lookup of kohu”. In Hawaiian Dictionary. Ulukau, the Hawaiian Electronic Library, University of Hawaii Press.
- Quivet E, Höhener P, Temime-Roussel B, Dron J, Revenko G, Verlande M, Lebaron K, Demales C, Vassalo L, Boudenne JL. 2022. Underestimation of anthropogenic bromoform released into the environment? Environ Sci Technol. 56(3):1522–1533. doi:10.1021/acs.est.1c05073.
- Risher J, Jones D, Lumpkin M. 2005. Toxicological profile for bromoform and dibromochloromethane. Atlanta, GA: Public Health Service: Agency for Toxic Substances and Disease Registry.
- Roque BM, Salwen JK, Kinley R, Kebreab E. 2019. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J Clean Prod. 234:132–138. doi:10.1016/j.jclepro.2019.06.193.
- Roque BM, Venegas M, Kinley R, de Nys R, Duarte T, Yang X, Salwen J, Kebreab E. 2021. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS One. 16(3):e0247820. doi:10.1371/journal.pone.0247820.
- Roskam E, Kirwan SF, Kenny DA, O’Donnell C, O’Flaherty V, Hayes M, Waters SM. 2022. Effect of brown and green seaweeds on diet digestibility, ruminal fermentation patterns and enteric methane emissions using the rumen simulation technique. Front Anim Sci. 3. doi:10.3389/fanim.2022.1021631.
- Rozman K, Hanninen O. 1986. Gastrointestinal toxicology. Amsterdam/New York: Elsevier Science Publishers; p. 543.
- Ruddick JA, Villeneuve DC, Chu I. 1983. A teratological assessment of four trihalomethanes in the rat. J Env Sci Health. 18:333–349. doi:10.1080/03601238309372373.
- Ryan S, Gleeson D, Jordan K, Furey A, O’Brien B. 2012. Evaluation of trichloromethane formation from chlorine-based cleaning and disinfection agents in cow’s milk. Int J Dairy Technol. 65(4):498–502. doi:10.1111/j.1471-0307.2012.00858.x.
- Sawyer MS, Hoover WH, Sniffen CJ. 1974. Effects of a ruminal methane inhibitor on growth and energy metabolism in the ovine. Journal of Animal Science. 38(4):908–914. doi:10.2527/jas1974.384908x.
- Shibazaki A, Ambiru K, Kurihara M, Tamegai H, Hashimoto S. 2016. Phytoplankton as a temperate marine source of brominated methanes. Marine Chemistry. 181:44–50. doi:10.1016/j.marchem.2016.03.004.
- Sigma-Aldrich. 2024. Safety data sheet bromoform version 6.7 revision date 07.03.2024 print date 24.03.2024. https://www.sigmaaldrich.com/NZ/en/sds/aldrich/241032.
- Stefenoni HA, Räisänen SE, Cueva SF, Wasson DE, Lage CFA, Melgar A, Fetter ME, Smith P, Hennessy M, Vecchiarelli B et al. 2021. Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J Dairy Sci. 104 (4) doi:10.3168/jds.2020-19686.
- Stevens JL, Anders MW. 1979. Metabolism of haloforms to carbon monoxide - III. Studies on the mechanism of the reaction. Biochemical Pharmacology. 28:3189–3194. doi:10.1016/0006-2952(79)90060-1.
- Stevens JL, Anders MW. 1981. Metabolism of haloforms to carbon monoxide. IV. Studies on the reaction mechanism in vivo. Chem Biol Interact. 37:365–374. doi:10.1016/0009-2797(81)90121-6.
- Sturges W, Cota G, Buckley P. 1992. Bromoform emission from Arctic ice algae. Nature. 358:660–662. doi:10.1038/358660a0.
- Sturges WT, Cota GF, Buckley PT. 1997. Vertical profiles of bromoform in snow, sea ice, and seawater in the Canadian Arctic. J Geophys Res Ocean. 102:25073–25083. doi:10.1029/97JC01860.
- Timbrell J, Barile FA. 2023. Introduction to toxicology. 4th ed. Boca Raton: CRC Press; p. 316 doi:10.1201/9781003188575.
- Tomkins NW, Colegate SM, Hunter RA. 2009. A bromochloromethane formulation reduces enteric methanogenesis in cattle fed grain-based diets. Anim Prod Sci. 49(12):1053–1058. doi:10.1071/EA08223.
- Ungerfeld EM. 2022. Opportunities and hurdles to the adoption and enhanced efficacy of feed additives towards pronounced mitigation of enteric methane emissions from ruminant livestock. Methane. 1(4):262–285. doi:10.3390/methane1040021.
- Ungerfeld EM, Beauchemin KA, Muñoz C. 2022. Current perspectives on achieving pronounced enteric methane mitigation from ruminant production. Front Anim Sci. 2:795200. doi:10.3389/fanim.2021.795200.
- United States Department of Health and Human Services. 1993. Hazardous substances data bank (HSDB, online database). National Toxicology Information Program, National Library of Medicine, Bethesda, MD.
- United States Environmental Protection Agency. 1989. http://water.epa.gov/resource_performance/performance/upload/OW_End_of_Year_BPFY2012_Report.pdf [accessed 2022 June].
- United States Environmental Protection Agency. 2017. Integrated risk information system (IRIS) on Bromoform. National Center for Environmental Assessment, Washington, D.C.
- Vaskoska RS. 2021. Raising a need for a risk assessment of bromoform transferred from feed to food. In: Food legal bulletin. Lawmedia Pty Ltd. https://www.foodlegal.com.au/inhouse/document/2440?print = true.
- von Oettingen WF. 1955. Bromoform. In: The Halogenated Aliphatic, Olefinic, Cyclic, Aromatic, and Aliphatic-Aromatic Hydrocarbons Including the Halogenated Insecticides, their Toxicity and Potential Dangers. U.S. Department of Health, Education and Welfare, Public Health Service, Washington, DC. USPHS Publ. No. 414, pp. 65-67.
- Vucko MJ, Magnusson M, Kinley RD, Villart C, De Nys R. 2017. The effects of processing on the in vitro antimethanogenic capacity and concentration of secondary metabolites of Asparagopsis taxiformis. J Appl Phycol. 29:1577–1586. doi: 10.1007/s10811-016-1004-3
- Wheeler T, Major R, Ogilvie S, South P, Romanazzi D, Adams S. 2021. Building a seaweed sector: developing a seaweed sector framework for Aotearoa New Zealand. Report for Sustainable Seas National Science Challenge. Project code. 2.5:45.
- WHO. 2012. Joint FAO/WHO expert meeting on dietary exposure assessment methodologies for residues of veterinary drugs: final report including report of stakeholder meeting. WHO, Geneva.
- WHO. 2022. Guidelines for drinking-water quality: fourth edition incorporating the first and second addenda. Geneva: WHO.
- Zhang X, Han Y, Huang W, Mingi J, Gao Z. 2021. The influence of gut microbiota on the bioavailability of oral drugs. Acta Pharm Sin B. 11(7):1789–1812. doi:10.1016/j.apsb.2020.09.013.
