Abstract
Phylogenetic and phenetic analyses of amplified fragment length polymorphism (AFLP) data were used to compare 38 individuals of 17 species of Australasian Wahlenbergia, with a focus on the New Zealand representatives. Overall, support values were slightly higher in the AFLP analyses than in previously published DNA sequence analyses, indicating the marginally better phylogenetic resolution of this technique. However, higher support was generally found towards the tips of the branches, whereas deeper relationships were equally poorly supported using either AFLPs or DNA sequence data. AFLP analyses are useful for species delimitation in New Zealand Wahlenbergia. The four members of the lowland radicate Wahlenbergia gracilis complex may all belong to the same species. We also present evidence that Wahlenbergia vernicosa should not be considered a subspecies of the Australian Wahlenbergia littoricola. The morphologically distinctive Wahlenbergia matthewsii and Wahlenbergia congesta subsp. haastii were recovered as being distinct using AFLP analyses, but members of the alpine rhizomatous Wahlenbergia albomarginata/ Wahlenbergia pygmaea complex could not be distinguished from each other.
Introduction
An interesting characteristic of the New Zealand flora is the relatively low level of interspecific sequence divergence in DNA regions that are commonly used in phylogenetic studies, even in groups with substantial morphological and ecological variation (Winkworth et al. Citation2005). This pattern is found across several New Zealand plant genera from both alpine and lowland areas (Wagstaff & Garnock-Jones Citation1998; Winkworth et al. Citation1999, Citation2002; Mitchell & Heenan Citation2002; Wagstaff et al. Citation2002; Meudt & Simpson Citation2006) and contributes to the difficulty in understanding species boundaries and evolutionary relationships in these groups.Footnote It also suggests that these species radiations have occurred relatively recently (Winkworth et al. Citation2005). Indeed, molecular clock analyses of nuclear ribosomal DNA (nrDNA) internal transcribed spacer (ITS) sequence data show that several genera of New Zealand plants have radiated within the last 5 Ma (Lockhart et al. Citation2001; Winkworth et al. Citation2002; Meudt et al. Citation2009; Tay et al. Citation2010; Prebble et al. Citation2011) during a period of rapid geological uplift and a changeable climate that created many new and fragmented habitats with fluctuating connectivity in New Zealand (Raven Citation1973). This may well have contributed to the pattern of low molecular vs. high morphological variation common in the New Zealand flora, as even dramatic morphological evolution may be the result of a small amount of genetic change at few loci (see references in Winkworth et al. Citation1999).
When low nrDNA and chloroplast (cp)DNA sequence variation results in poorly resolved phylogenies, the amplified fragment length polymorphism (AFLP) approach has the potential to overcome such difficulties (Meudt & Clarke Citation2007). This simultaneous analysis of many AFLP loci sampled predominantly across the nuclear genome is also more likely to generate a species tree, rather than generating individual gene trees as in DNA sequencing (Després et al. Citation2003). Several recent studies have turned to AFLP data to overcome the problems associated with low DNA sequence variation in the New Zealand flora (Perrie et al. Citation2003; Meudt & Bayly Citation2008; Broadhurst et al. Citation2008; Smissen & Breitwieser Citation2008; Meudt et al. Citation2009; Perrie & Shepherd Citation2009; Meudt Citation2011).
New Zealand species of Wahlenbergia Schrader ex. Roth (Campanulaceae) (Petterson Citation1997a) are a prime candidate for analysis with AFLP data to resolve species relationships and test species limits because their relationships were not well resolved using DNA sequence data (Prebble et al. Citation2011, Citation2012). In the first global phylogeny of the genus, Prebble et al. (Citation2011) showed that the ancestors of New Zealand Wahlenbergia probably arrived in New Zealand from Australia and then radiated during the last 1.6 Ma. There were two clear lineages present, corresponding to the radicate and rhizomatous growth forms (Prebble et al. Citation2011). In a follow-up study focusing on the Australasian species, Prebble et al. (Citation2012) encountered low sequence variation of ITS and two cpDNA loci (trnL-F and trnK), which created difficulties in resolving the relationships of several of the species within the radicate and rhizomatous lineages. Of the five New Zealand radicate species (Wahlenbergia akaroa J.A.Petterson, Wahlenbergia ramosa G.Simpson, Wahlenbergia rupestris G. Simpson, Wahlenbergia vernicosa J. A. Petterson and Wahlenbergia violacea J. A. Petterson), W. vernicosa has a unique chromosome number (2n=54, vs 2n=72 for all other New Zealand radicate species [Petterson et al. Citation1995]), and there is evidence from cpDNA sequence data that it forms a distinct lineage (Prebble et al. Citation2012). However, DNA sequence data do not falsify the hypothesis that the other four radicate species (hereafter referred to as the Wahlenbergia gracilis complex, following Webb & Simpson Citation2001) are conspecific (Prebble et al. Citation2012). De Lange and Cameron (Citation1999) suggested that W. vernicosa should be treated as a subspecies of the Australian Wahlenbergia littoricola (as W. littoricola subsp. vernicosa) based on the morphological similarities of those two entities. However, DNA sequence data did not support that hypothesis. cpDNA and nrDNA sequence data suggest that the five New Zealand rhizomatous species form a monophyletic group, which indicates radiation following a single introduction from Australia (Prebble et al. Citation2012). In the rhizomatous group, Prebble et al. (Citation2012) found that three morphologically distinctive species had some level of genetic distinction (Wahlenbergia cartilaginea Hook.f., Wahlenbergia matthewsii Cockayne and Wahlenbergia congesta (Cheeseman) N.E.Br.), which confirms their present taxonomic recognition as separate species. The other two species (Wahlenbergia albomarginata Hook. and Wahlenbergia pygmaea Colenso) were largely genetically indistinguishable (with the exception of W. albomarginata subsp. olivina J.A.Petterson). Prebble et al. (Citation2012) concluded that although some of the species hypotheses (Smith Citation1992; Petterson Citation1997a; de Lange & Cameron Citation1999; Plunkett et al. Citation2009) were supported, a larger number could not be adequately addressed by the DNA sequence dataset, and that AFLP data could be useful.
In this study, we ask whether AFLP data will be useful to: (1) explore species limits in the W. gracilis complex, (2) clarify the taxonomic rank of the New Zealand radicate W. vernicosa relative to the Australian W. littoricola, and (3) assess species monophyly of the rhizomatous New Zealand Wahlenbergia. We follow de Queiroz (Citation2007) in defining species as separately evolving metapopulation lineages that are demographically and genetically interconnected sets of populations with continuity over time (Mayden Citation1997). Although this definition does not provide an unambiguous cut off for when speciation has occurred, it emphasizes that the primary goal of species delimitation research is lineage discovery and delimitation by using evidence from numerous sources of data, such as morphology, cytology, DNA sequence and high resolution markers. In this article, we draw on evidence from AFLP data (this study), DNA sequence data (Prebble et al. Citation2011, Citation2012), morphological data (Petterson Citation1997a) and cytological data (Petterson et al. Citation1995). We treat the current taxonomy as hypotheses to be tested, and assume that the null hypothesis is that of conspecifity between all the taxa considered pairwise, hence the onus is on finding lines of evidence that compel rejection of the null hypothesis.
Methods
Sampling and DNA extraction
In total, 38 individuals from 17 species were chosen for AFLP analysis: 31 New Zealand samples and 7 Australian samples (). These individuals were selected to represent each New Zealand species and subspecies. We note that the sampling in this article is somewhat sparser than would usually be recommended for a study of this type. This is because we were limited to working with samples that had already been collected for a previous DNA-sequencing study (Prebble et al. Citation2012). Within this limitation, we sampled as fully as we were able, taking multiple samples from the same population and/or from different populations of the same species where possible. For example, for W. albomarginata we included nine samples—four of the widespread W. albomarginata subsp. albomarginata (from throughout its range in South Island alpine areas), two each of two of the geographically restricted subspecies (W. albomarginata subspp. olivina and flexilis), but only one of the possibly fairly widespread W. albomarginata subsp. laxa.
Table 1 Voucher information for each of the 38 individuals of Wahlenbergia included in the AFLP study.
Total DNA was extracted from silica-dried leaves after manual disruption of dried tissue with a mortar and pestle using a CTAB method modified from Doyle & Doyle (Citation1990). Only DNA of high quality and high concentration was used. Quality was determined by viewing the extracted DNA on a 1.5% agarose gel and quantity was assessed by use of a NanoDrop™ ND-1000 spectrophotometer (Thermo Fisher Scientific New Zealand, Auckland). Only samples with concentrations of DNA > 250 ng/µL were used.
AFLP data generation and automated scoring
AFLPs were generated based on the protocol of Vos et al. (Citation1995) using an updated protocol for restriction enzyme digestion with EcoRI (Roche, Auckland, New Zealand) and MseI (NEB, via Thermo Fisher Scientific New Zealand), ligation, pre-selective amplification, selective amplification with fluorescently labelled markers and capillary detection of fluorescently labelled markers (http://www.clarkeresearch.org/aflp/; Meudt & Bayly Citation2008). For samples with a concentration > 1000 ng/µL, 3 µL of DNA was digested, and for samples < 1000 ng/µL, 5 µL was digested. This was done to ensure a similar amount of DNA in each sample. For selective amplification, four different fluorescently labelled Eco+ANN primers were used and trialled with a selection of potential combinations of eight different Mse+CNN primers. The following primer combinations were chosen based on a screening involving four individuals: 6-FAM–Eco+ACT/Mse+CAA, VIC–Eco+ATA/Mse+CGT, NED–Eco+ACC/Mse+CAC and PET–Eco+AGG/Mse+CAA (here after, 6-FAM, VIC, NED, PET). All primers were supplied by Sigma (Auckland, New Zealand) except VIC-, NED- and PET-labelled primers (Applied Biosystems, Auckland, New Zealand). For each individual, selective amplifications of each of the four dyes were mixed together in the ratio of 1:1:1:2 (6-FAM/VIC/NED/PET), along with a GS-500 LIZ size standard, and 1 µL of each sample was run on an Applied Biosystems Genetic Analyzer (ABI3730) at the Allan Wilson Centre Genome Service (Massey University, Palmerston North, New Zealand). To ascertain reproducibility, replicate AFLP profiles were generated from independent restriction digests of the same DNA extraction for six individuals from six different species (c. 10% of the dataset; see ). Owing to limited material, we did not include replicate DNA extractions of the same sample. Replicates were included in different runs on the 3730 Genetic Analyzer to ensure that samples in different runs were comparable.
Automated scoring was performed on the resulting electronic AFLP profiles from all four dyes (and again with three dyes only, excluding 6-FAM due to several failed samples for that dye) using GeneMarker v 1.80 (SoftGenetics, State College, PA, USA). Scoring parameters were optimized following the procedure developed by Holland et al. (Citation2008) and are discussed in more detail in Prebble (Citation2010). Thirty-six different Wahlenbergia AFLP character matrices were generated in GeneMarker that varied by setting peak height threshold (PHT), minimum fragment length (MFL), stutter peak filter (SPF), and local and global detection percentages (LGDP). Comparisons of the 36 datasets to determine optimal scoring parameter settings were streamlined using the Python scripts of Holland et al. (Citation2008) to analyse the PAUP* output and produce several measures of accuracy.
Phylogenetic and principal coordinate analyses of AFLP data
Phylogenetic trees were reconstructed using maximum parsimony (MP) and Bayesian inference. MP trees were built using PAUP* v4b10 (Swofford, Citation2002). The MP analyses were performed in a two-step search strategy. First, multiple islands were searched with 10,000 random addition sequence replicates, nchuck = 5, chuckscore = 1 and maxtrees = 10,000. The resulting trees were then swapped to completion with the same settings but chuckscore = no. Support for clades was assessed using 1000 bootstrap replicates, 10 random addition replicates and MAXTREES =20,000 in PAUP*. For Bayesian inference, a restriction site (binary) model was used (Luo et al. Citation2007). Although the evolution of AFLP markers is far more complex than the model assumes, this model has been shown to be useful for analysing AFLP data (Koopman et al. Citation2008). Four independent chains with 10 million iterations were run using MrBayes v 3.1.2 (Ronquist & Huelsenbeck Citation2003). Convergence was assumed to have occurred when the standard deviation of split frequencies was below 0.01. The output was analysed in Tracer v1.4.1 (Rambaut & Drummond Citation2007) to determine the point at which the analysis had reached stationarity; a burn in of 5% was thus chosen.
The program NTSYS-pc (Rohlf Citation1990) was used to conduct a principal coordinate analysis (PCoA) to investigate clusters in the dataset. PCoA is an ordination method, which graphically explores the similarities within the data by mapping a similarity matrix. A PCoA allows the assessment of the dimensionality of the data and a description of the major patterns of variation within and between populations (Ishida et al. Citation2003). The PCoA was performed using Jaccard distances, DCENTER, EIGEN and MOD3D modules with three dimensions analysed and visualized. Initially, the full dataset was analysed, followed by subsets of the dataset to further investigate clusters of interest. Principal coordinate model clustering (PCO-MC) was used to test the significance of clusters, using NTSYS and the MODECLUS procedure in SAS 9.1 (SAS Institute, Cary, NC) following Reeves & Richards (Citation2009). PROC MODECLUS was used to perform a saddle test to determine whether a given cluster was significantly distinct. Details of the saddle test are available in the SAS manual (Chapter 42, The MODECLUS Procedure, SAS Institute, Cary, NC).
Results
Of the 36 datasets generated for AFLP scoring parameter optimization, those with the greatest number of characters also had the largest number of parsimony informative characters and the highest resolution scores. Unfortunately, they also had the highest error rates and the lowest normalized resolution scores. Nevertheless, in general, the datasets showed little variability with respect to the measures of accuracy tested (data not shown, see Prebble, Citation2010 for details).
The small amount of variation in all accuracy measures, as well as a lack of reticulation in the majority rule consensus network (data not shown; see Prebble Citation2010) suggests that this particular dataset is relatively robust to changes in the scoring parameters, and any of the datasets could be selected for downstream analyses. Therefore, we selected the dataset with the lowest Euclidean error rate (7%), highest normalized resolution score (87%) and best replicate pairing (Prebble Citation2010). The optimal scoring parameters were: PHT, 150; MFL, 100; SPF, on; and LGDP, off. The final AFLP dataset contained 715 characters from three primer combinations (excluding 6-FAM), 555 of which were parsimony informative.
The Bayesian phylogeny () is shown as an unrooted tree, because it is unclear which species should be at the root (Prebble et al. Citation2012). The 50% majority rule tree of the MP analysis (of five most parsimonious trees; Supplementary information) was topologically similar to the Bayesian tree, though with some differences. For example, the W. vernicosa samples (excluding W.vernicosa5_Raoul) formed a monophyletic group in the MP analysis (< 50% BS), but in the Bayesian analysis the W. vernicosa sample from the Chatham Islands (W.vernicosa1_Chatham) formed a clade with the W. violacea sample (W.violacea1_Chatham) also from the Chatham Islands (0.89 PP). Both methods grouped the New Zealand rhizomatous species into a poorly supported clade (0.77 PP, 51% BS; ). A New Zealand radicate clade (excluding W.vernicosa5_Raoul) was highly supported in the Bayesian analysis (0.97 PP, ). The MP analysis recovered an unsupported clade containing all of the New Zealand radicate species plus the Australian radicate sample W.gracilis2_NSW (Supplementary information). The position of the Australian samples was unresolved between the New Zealand rhizomatous and New Zealand radicate groups in both analyses. Higher support values were found usually on branches connecting conspecific terminal taxa.
Figure 1 Bayesian 50% majority rule consensus tree of Australasian Wahlenbergia based on the AFLP dataset. Numbers near branches are posterior probability (PP) values/ bootstrap (BS) values from the MP analysis. *Indicates support of less than 0.5 PP or 50% BS for that branch. See for an explanation of the tag names and voucher information.
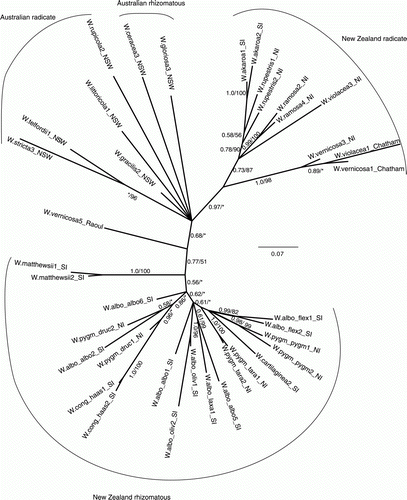
Wherever multiple populations were sampled (radicate species: W. rupestris, W. vernicosa and W. violacea; rhizomatous species: W. albomarginata and W. pygmaea), species were not monophyletic. The samples of New Zealand W. vernicosa (W. littoricola subsp. vernicosa sensu de Lange & Cameron Citation1999) did not group with the Australian W. littoricola sample.
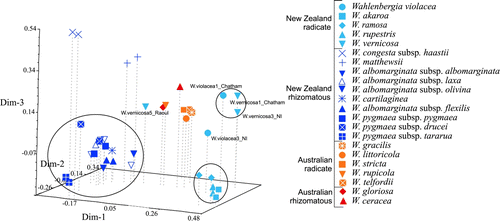
The PCoA revealed similar patterns to the tree building methods, and the PCO-MC analysis identified three clusters as significant (). The first three dimensions explained 9.4%, 5.3% and 4.8% of the variation, respectively. Dimensions 1 and 2 separated the New Zealand radicate species, whereas dimensions 1 and 3 separated the New Zealand rhizomatous species, particularly W. matthewsii and W. congesta subsp. haastii. Individuals of W. albomarginata, W. pygmaea and W. cartilaginea were not significantly separated from each other ().
Figure 2 Principal coordinates analysis (PCoA) of Australasian Wahlenbergia AFLP data set showing three dimensions. Dimension one explains 9.4%, dimension two an additional 5.3% and dimension three a further 4.8%. Clusters found to be significant using PCO-MC are circled. Explanation of tag names and information on voucher specimens can be found in .
Discussion
AFLPs are useful markers to explore species limits, clarify taxonomy and assess monophyly of New Zealand Wahlenbergia, as demonstrated by the generally resolved MP and Bayesian phylogenies, which contain several well-supported lineages, and the presence of significantly separated PCoA clusters. Overall, similar patterns of relationships were found with AFLP analyses relative to those found using DNA sequence analyses (Prebble et al. Citation2012), but support values—which can be used as a proxy for level of resolution within the data (Holland et al. Citation2008)—were slightly higher in the AFLP analyses, especially towards the tips of the trees. Given the low support for branches deeper in the trees, it is apparent that AFLPs are more useful at testing species and subspecies delimitation than at assessing phylogenetic relationships, which is consistent with previous studies (Mueller & Wolfenbarger Citation1999; Meudt & Clarke Citation2007; Meudt et al. Citation2009; Meudt Citation2011).
AFLP data are generally more useful at the lower taxonomic levels where there is usually lower genetic divergence between samples, because homoplasy becomes a problem as genetic diversity increases between samples (Meudt & Clarke Citation2007). García-Pereira et al. (Citation2010) used simulation studies to demonstrate that AFLP data contain too many non-homologous fragments to be able to accurately reconstruct phylogenies when DNA sequence divergence is greater than c. 0.05 substitutions per site. These results are congruent with results from analyses of real datasets that show that non-homology in AFLP datasets can become an issue when ITS sequence divergence is greater than c. 30–35 nucleotide differences (i.e. 35 of c. 700 total ITS sites = 0.05) (Koopman Citation2005). Prebble et al. (Citation2012) showed that divergence (given as uncorrected p distances of ITS and trnK–psbA sequence data, see Table 4 in Prebble et al. Citation2012) between samples within the New Zealand radicate and rhizomatous Wahlenbergia species was < 0.05, but that the divergence within radicate and rhizomatous Australian species was >0.05. This fact, together with the similarity of the results from the PCoA, MP and Bayesian analyses, suggests that saturation (caused, for example, by the non-homologous nature of some of the shared absence of AFLP bands) is not an issue regarding phylogeny and species delimitation of the New Zealand species. However, relationships between the Australian species are completely unresolved (), which may be due to homoplasy caused by their greater divergence.
Species limits in the W. gracilis complex
Overall there is little evidence from AFLP (this study) and DNA sequence data (Prebble et al. Citation2012) or morphology (Petterson Citation1997a) for speciation within the W. gracilis complex (W. akaroa, W. ramosa, W. rupestris and W. violacea; see ). However, the lack of phylogenetic signal in the AFLP and DNA sequence data may simply reflect the recent evolution of the New Zealand radicate species (ca. 0.7 Ma; Prebble et al. Citation2011), such that insufficient time has passed for reciprocal species monophyly to have evolved (Shaffer & Thomson Citation2007). Therefore, further tests with wider sampling, including multiple representatives from multiple populations of each currently recognized species are warranted. A systematic assessment of the morphological variation present in this complex (including rigorous morphometrics of flower size [Petterson Citation1997a] and other characters) is also key for determining the validity of these species. Nevertheless, based on the data we have collected so far, we recommend the W. gracilis complex should be considered one species. However, we are uncertain whether these New Zealand plants are conspecific with W. gracilis (G.Forst.) A.DC. and further research is needed (see Petterson Citation1997b).
Table 2 Synthesis table, showing relationships found among samples of Australian and New Zealand Wahlenbergia for the same morphological species analysed in Prebble et al. (Citation2011) and this study.
The taxonomic rank of the New Zealand radicate W. vernicosa relative to the Australian W. littoricola
The AFLP (this study) and DNA sequence analyses (Prebble et al. Citation2012) do not support a close relationship between any of the individuals of W. vernicosa from New Zealand and the W. littoricola samples from Australia. These results therefore falsify the hypothesis (de Lange and Cameron Citation1999) that these species are conspecific (see ). We therefore recommend recognizing W. vernicosa at species rank. Some interesting aspects of W. vernicosa require further study. For example, the assumption that W. vernicosa does not form part of the W. gracilis complex on the basis of chromosome counts requires further investigation as at this stage only two counts of W. vernicosa are reported (Petterson et al. Citation1995). As there are several cases of chromosome races occurring within Australian radicate species (Smith Citation1992), a greater number of chromosome counts is needed. Additionally, the placement of the Raoul Island Wahlenbergia sample is problematic; morphologically it appears to be W. vernicosa, yet it grouped more closely with Australian Wahlenbergia than with the other two W. vernicosa samples (–). Further sampling and chromosome counts of the Raoul Island plants will help establish their identity, and to determine if they are conspecific with the New Zealand samples.
Species monophyly of the rhizomatous New Zealand Wahlenbergia
The New Zealand rhizomatous species form a poorly supported clade in the AFLP trees (0.77 PP, 51% BS) and a moderately supported clade in the results of the DNA sequence analyses (Prebble et al. Citation2012). Within this clade, W. matthewsii and W. congesta form distinct lineages (–; Prebble et al. Citation2012). Morphological characters provide additional evidence for their recognition at species rank (Table 2), i.e. W. matthewsii has a different growth form (suffructose rhizomatous) that distinguishes it from all other New Zealand Wahlenbergia species, and W. congesta has distinctive morphological characters including sessile, glossy, spathulate leaves, subsessile flowers and spherical capsules (Petterson Citation1997a).
The phylogenetic AFLP analyses were unable to resolve the relationships of the single W. cartilaginea sample (), although it did group within the statistically significant W. albomarginata/W. pygmaea PCoA cluster (). However, the species’ distinct morphology (Petterson Citation1997a) and scree habitat, and its monophyly in phylogenies reconstructed from both nrDNA and cpDNA sequence data (Prebble et al. Citation2012), compel rejection of a hypothesis of conspecificity with the other New Zealand rhizomatous species.
Accessions of W. albomarginata and W. pygmaea also did not form clades in the AFLP phylogenetic analyses. Therefore, like the DNA sequence analyses of Prebble et al. (Citation2012), the AFLP data did not provide evidence to refute the hypothesis that W. albomarginata and W. pygmaea are conspecific (Prebble et al. Citation2012). As in the DNA sequence analyses, individuals of the same subspecies collected from the same location generally grouped together, but when samples from other populations were included, subspecies were not monophyletic (e.g. W. albomarginata subsp. albomarginata). Nevertheless, as shown by the PCoA (), AFLPs are potentially a useful technique for exploring the relationship between W. albomarginata and W. pygmaea in a future study that uses increased sampling to represent different populations of all subspecies from throughout their ranges.
Conclusions
We have shown that AFLPs provide a useful technique for delimiting species in New Zealand Wahlenbergia. We have been able to address several species hypotheses more fully than by using DNA sequence data. However, it is clear that a more comprehensive sampling strategy will be essential in order to address the outstanding questions in this group. Moreover, it is possible that even with greater sampling these questions will still not be adequately addressed; species delimitation of recently evolved species can be problematic even when using many lines of evidence, including high-resolution markers (Shaffer and Thompson Citation2007), especially as the criterion of lineage monophyly may not be met for a variety of reasons. These kinds of molecular techniques rely on random mutations, and the probability that such mutations are able to correctly assign each individual to a species group must be relatively low due to the recent evolution of Wahlenbergia in New Zealand (ca. 1.6 my, Prebble et al. Citation2011). Thus, some level of fuzziness and uncertainty in delimiting young species as found here is perhaps to be expected (Shaffer & Thompson Citation2007). Consequently, recent evolution and species radiation, such as that occurring within Australasian Wahlenbergia, continues to present a challenge to systematists and taxonomists.
Supplementary file
Supplementary file: Maximum parsimony Wahlenbergia AFLP tree
tnzb_a_698624_sup_26746379.pdf
Download PDF (166.8 KB)Acknowledgements
We thank Lesley Bagnall, Mike Bayly, Jeremy Bruhl, Ewen Cameron, Hamish Carson, Peter de Lange, Teresa Herleth, Fiona Hodge, George Plunkett, Mark Prebble, Gesine Pufal, Tony Silbery, Ian Telford, and Hugh Wilson for help with collecting. Three anonymous reviewers provided helpful comments on an earlier draft. We gratefully acknowledge financial support from the Auckland Botanical Society (Lucy Cranwell field grant), Museum of New Zealand Te Papa Tongarewa (Te Papa MSc Scholarship in Molecular Systematics) and Victoria University of Wellington (Masters Scholarship and research funding).
Notes
Supplementary data available online at www.tandfonline.com/10.1080/0028825X.2012.698624
Supplementary file: Maximum parsimony Wahlenbergia AFLP tree
References
- Broadhurst , LM , Young , AG and Murray , BG . 2008 . AFLPs reveal an absence of geographical genetic structure among remnant populations of pohutukawa (Metrosideros excelsa, Myrtaceae) . New Zealand Journal of Botany , 46 : 13 – 21 .
- de Lange , PJ and Cameron , EK . 1999 . The vascular flora of Aorangi Island, Poor Knights Islands, northern New Zealand . New Zealand Journal of Botany , 37 : 433 – 468 .
- de Queiroz , K . 2007 . Species concepts and species delimitation . Systematic Biology , 56 : 879 – 886 .
- Després , L , Gielly , L , Redoutet , W and Taberlet , P . 2003 . Using AFLP to resolve phylogenetic relationships in a morphologically diversified plant species complex when nuclear and chloroplast sequences fail to reveal variability . Molecular Phylogenetics and Evolution , 27 : 185 – 196 .
- Doyle , JJ and Doyle , JL . 1990 . Isolation of plant DNA from fresh tissue . Focus , 12 : 13 – 15 .
- García-Pereira , MJ , Caballero , A and Quesada , H . 2010 . Evaluating the relationship between evolutionary divergence and phylogenetic accuracy in AFLP data sets . Molecular Biology and Evolution , 27 : 988 – 1000 .
- Holland , B , Clarke , AC and Meudt , HM . 2008 . Optimizing automated AFLP scoring parameters to improve phylogenetic resolution . Systematic Biology , 57 : 347 – 366 .
- Ishida , TA , Hattori , K , Sato , H and Kimura , MT . 2003 . Differentiation and hybridization between Quercus crispula and Q. dentata (Fagaceae): insights from morphological traits, amplified fragment length polymorphism markers, and leafminer composition . American Journal of Botany , 90 : 769 – 776 .
- Koopman , WJM . 2005 . Phylogenetic signal in AFLP data sets . Systematic Biology , 54 : 197 – 217 .
- Koopman , WJM , Wissemann , V , De Cock , K , Van Huylenbroeck , J , De Riek , J , Sabatlno , GJH , Visser , D , Vosman , B , Ritz , CM , Maes , B , Werlemark , G , Nybom , H , Debener , T , Linde , M and Smulders , MJM . 2008 . AFLP markers as a tool to reconstruct complex relationships: a case study in Rosa (Rosaceae) . American Journal of Botany , 95 : 353 – 366 .
- Lockhart , PJ , McLenachan , PA , Havell , D , Glenny , D , Huson , D and Jensen , U . 2001 . Phylogeny, radiation, and transoceanic dispersal of New Zealand alpine buttercups: molecular evidence under split decomposition . Annals of the Missouri Botanical Garden , 88 : 458 – 477 .
- Luo , RY , Hipp , AL and Larget , B . 2007 . A Bayesian model of AFLP marker evolution and phylogenetic inference . Statistical Applications in Genetics and Molecular Biology , 6 : 1 – 32 .
- Mayden , RL . 1997 . “ A hierarchy of species concepts: the denouement in the saga of the species problem ” . In Species: The units of biodiversity , Edited by: Claridge , MF , Dawah , HA and Wilson , MR . 381 – 424 . London : Chapman and Hall .
- Meudt , HM . 2011 . AFLP data reveal a history of auto- and allopolyploidy in New Zealand endemic species of Plantago (Plantaginaceae): new perspectives on a taxonomically challenging group . International Journal of Plant Sciences , 172 : 220 – 237 .
- Meudt , HM and Bayly , MJ . 2008 . Phylogeographic patterns in the Australasian genus Chionohebe (Veronica s.l., Plantaginaceae) based on AFLP and chloroplast DNA sequences . Molecular Phylogenetics and Evolution , 47 : 319 – 338 .
- Meudt , HM and Clarke , AC . 2007 . Almost forgotten or latest practice? AFLP applications, analyses and advances . Trends in Plant Science , 12 : 106 – 117 .
- Meudt , HM , Lockhart , PJ and Bryant , D . 2009 . Species delimitation and phylogeny of a New Zealand plant species radiation . BMC Evolutionary Biology , 9 : 111
- Meudt , HM and Simpson , BB . 2006 . The biogeography of the austral, subalpine genus Ourisia (Plantaginaceae) based on molecular phylogenetic evidence: South American origin and dispersal to New Zealand and Tasmania . Biological Journal of the Linnean Society , 87 : 479 – 513 .
- Mitchell , AD and Heenan , PB . 2002 . Sophora sect. Edwardsia (Fabaceae): further evidence from nrDNA sequence data of a recent and rapid radiation around the southern oceans . Botanical Journal of the Linnean Society , 140 : 435 – 441 .
- Mueller , UG and Wolfenbarger , LL . 1999 . AFLP genotyping and fingerprinting . Trends in Ecology & Evolution , 14 : 389 – 394 .
- Perrie , LR , Brownsey , PJ , Lockhart , PJ , Brown , EA and Large , MF . 2003 . Biogeography of temperate Australasian Polystichum ferns as inferred from chloroplast sequence and AFLP . Journal of Biogeography , 30 : 1729 – 1736 .
- Perrie , LR and Shepherd , LD . 2009 . Reconstructing the species phylogeny of Pseudopanax (Araliaceae), a genus of hybridising trees . Molecular Phylogenetics and Evolution , 52 : 774 – 783 .
- Petterson , JA . 1997a . Revision of the genus Wahlenbergia (Campanulaceae) in New Zealand . New Zealand Journal of Botany , 35 : 9 – 54 .
- Petterson , JA . 1997b . Identity of the original Wahlenbergia gracilis (Campanulaceae) and allied species. An historical review of early collections . New Zealand Journal of Botany , 35 ( 1 ) : 55 – 78 .
- Petterson , JA , Williams , EG and Dawson , MI . 1995 . Contributions to a chromosome atlas of the New Zealand flora—34. Wahlenbergia (Campanulaceae) . New Zealand Journal of Botany , 33 : 489 – 496 .
- Plunkett , GT , Bruhl , JJ and Telford , IRH . 2009 . Two new, sympatric species of Wahlenbergia (Campanulaceae) from the New England Tableland escarpment, New South Wales, Australia . Australian Systematic Botany , 22 : 319 – 331 .
- Prebble JM 2010 . The evolution of Wahlenbergia (Campanulaceae) in Australasia. Unpublished Masters thesis . Victoria University of Wellington , New Zealand . http://researcharchive.vuw.ac.nz//handle/10063/1338 (accessed 3 August 2012).
- Prebble , JM , Cupido , CN , Meudt , HM and Garnock-Jones , PJ . 2011 . First phylogenetic and biogeographical study of the southern bluebells (Wahlenbergia, Campanulaceae) . Molecular Phylogenetics and Evolution , 59 : 636 – 648 .
- Prebble , JM , Meudt , HM and Garnock-Jones , PJ. 2012 . An expanded molecular phylogeny of the southern bluebells (Wahlenbergia, Campanulaceae) from Australia and New Zealand . Australian Systematic Botany , 25 : 11 – 30 .
- Rambaut A , Drummond AJ 2007 . Tracer v1.4 . http://beast.bio.ed.ac.uk/Tracer (accessed 3 August 2012).
- Raven , PH . 1973 . Evolution of the subalpine and alpine plant groups in New Zealand . New Zealand Journal of Botany , 11 : 177 – 200 .
- Reeves , PA and Richards , CM . 2009 . Accurate inference of subtle population structure (and other genetic discontinuities) using principal coordinates . PLoS ONE , 4 : e4269
- Rohlf FJ 1990 . NTSYS-pc: numerical taxonomy and multivariate analysis system . Applied Biostatistics , Setauket , NY .
- Ronquist , F and Huelsenbeck , JP . 2003 . MRBAYES 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Shaffer , H and Thomson , R . 2007 . Delimiting species in recent radiations . Systematic Biology , 56 : 896 – 906 .
- Smissen , R and Breitwieser , I . 2008 . Species relationships and genetic variation in the New Zealand endemic Leucogenes (Asteraceae: Gnaphalieae) . New Zealand Journal of Botany , 46 : 65 – 76 .
- Smith , PJ . 1992 . A revision of the genus Wahlenbergia (Campanulaceae) in Australia . Telopea , 5 : 91 – 175 .
- Swofford DL 2002 . PAUP* . Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4 . Sinauer Associates , Sunderland , MA .
- Tay , ML , Meudt , HM , Garnock-Jones , PJ and Ritchie , PA . 2010 . DNA sequences from three genomes reveal multiple long-distance dispersals and nonmonophyly of sections in Australasian Plantago (Plantaginaceae) . Australian Systematic Botany , 23 : 47 – 68 .
- Vos , P , Hogers , R , Bleeker , M , Reijans , M , Vandelee , T , Hornes , M , Frijters , A , Pot , J , Peleman , J , Kuiper , M and Zabeau , M . 1995 . AFLP—a new technique for DNA-fingerprinting . Nucleic Acids Research , 23 : 4407 – 4414 .
- Wagstaff , SJ , Bayly , MJ and Garnock-Jones , PJ . 2002 . Classification, origin, and diversification of the New Zealand hebes (Scrophulariaceae) . Annals of the Missouri Botanical Garden , 89 : 38 – 63 .
- Wagstaff , SJ and Garnock-Jones , PJ . 1998 . Evolution and biogeography of the Hebe complex (Scrophulariaceae) inferred from ITS sequences . New Zealand Journal of Botany , 36 : 425 – 437 .
- Webb , CJ and Simpson , MJA . 2001 . Seeds of New Zealand gymnosperms and dicotyledons , 154 – 156 . Christchurch : Manuka Press .
- Winkworth , RC , Grau , J , Robertson , A and Lockhart , PJ . 2002 . The origins and evolution of the genus Myosotis L. (Boraginaceae) . Molecular Phylogenetics and Evolution , 24 : 180 – 193 .
- Winkworth , RC , Robertson , AW and Ehrendorfer , F . 1999 . The importance of dispersal and recent speciation in the flora of New Zealand . Journal of Biogeography , 26 : 1323 – 1325 .
- Winkworth , RC , Wagstaff , SJ and Glenny , D . 2005 . Evolution of the New Zealand mountain flora: origins, diversification and dispersal . Organisms Diversity & Evolution. , 5 : 237 – 247 .