ABSTRACT
Fire regimes are powerful selective filters. In New Zealand, fire activity was rare before human settlement, and New Zealand’s indigenous woody flora shows little adaptation to frequent fire. One of the few woody indigenous species to show adaptation to frequent fire is Leptospermum scoparium (Myrtaceae), a Plio-Pleistocene immigrant from Australia. Leptospermum scoparium is a widespread early-successional shrub to small tree that frequently dominates post-fire successions, and shows geographically-variable pyriscent serotiny, a fire-adaptation in which seeds are retained in the canopy and open post-fire. Another widespread reproductive adaptation to fire is heat-stimulated germination. Some Australian Leptospermum respond positively to heat treatment, and observations in New Zealand suggest that the seeds of L. scoparium within thick-walled capsules open immediately after fire and successfully germinate, indicating at least no deleterious effect of heating. In some fire-prone systems such as Mediterranean shrublands, traits such as serotiny and heat-stimulated germination have been positively associated with traits associated with flammability (e.g. retention of dead fuel). Here we evaluate the effect of heat stimulation (short exposure to high temperatures) of capsules on germination in L. scoparium from New Zealand, and evaluate links between germination, serotiny and shoot-level flammability. Germination trials using seed collected from 12 populations indicated no consistent positive or negative effect of heat treatment on germination success (germinability). These trials suggest that the capsules of L. scoparium at least provide adequate insulation to heating. Germinability was not consistently related to either serotiny or flammability; nor was it related to latitude or elevation. While taxa from other fire-prone ecosystems may show coordinated trait responses to fire, the lack of association between physical measures of flammability and germination rates indicate that this is not the case in L. scoparium, New Zealand’s most fire-adapted indigenous woody species.
Introduction
Plants show a range of reproductive adaptations to fire regimes, including canopy seed storage with release after fire (pyriscent serotiny), smoke and/or heat stimulated dormancy breaking, and germination enhancement (Keeley and Fotheringham Citation2000; Keeley et al. Citation2011). Pyriscent serotiny is a widespread adaptation among ‘seeder’ species, which are killed by fire but store large amounts of seed between such events and then release them after fire (Lamont et al. Citation1991). Most studies of fire-related traits have considered ecosystems where fire is frequent, or species that are frequently burned. However, flammable taxa and some fire-adaptive traits are also found in settings where fire is rare (Bowman et al. Citation2014; Wyse et al. Citation2016), suggesting that directly linking flammability, fire regime and fire-associated traits is not always straightforward (Richardson et al. Citation2015). Functional traits are not independent of each other but co-vary within and between species and with the environment (Westoby et al. Citation2002; Dwyer and Laughlin Citation2017). In the specific context of fire, for example, elevated flammability should provide pyriscent serotinous species with an advantage in terms of seed release (Schwilk and Ackerly Citation2001). Pausas and Moreira (Citation2012) reason that flammability should be coordinated with other fire-associated reproductive traits, such as heat-triggered dormancy breaking and heat-stimulated germination. Examples of this trait coordination are found in some Pinus (He et al. Citation2012) and some Banksia (He et al. Citation2011) where flammability (as indicated by the retention of dead fine fuels) is associated with the presence of serotiny. Similarly, Delgado et al. (Citation2001) describe complex trade-offs between heating effects, germination success, seed mass and seed production in the fire-prone shrub Cistus ladanifer (Cistaceae). In this species, seed mass is negatively correlated with seed production (as expected); however, larger seeds have a higher germination rate if subjected to heating. The outcome of this interaction is that the production of many small seeds is favoured in environments where fire activity is limited and vice versa (Delgado et al. Citation2001).
New Zealand provides a key example of the complexity in the links between flammability, fire frequency and fire traits. Natural fire was rare in most New Zealand ecosystems throughout the Holocene until the recent arrival of people c. 1280 AD (Wilmshurst et al. Citation2008), after which time widespread deforestation by fire ensued (Ogden et al. Citation1998; McGlone and Wilmshurst Citation1999; Perry et al. Citation2014). However, despite the rarity of natural fire prior to human settlement, some indigenous plant species are highly flammable (Wyse et al. Citation2016) and large areas of woody vegetation were rapidly destroyed following the onset of anthropogenic fire (McWethy et al. Citation2014). There are very few fire-adapted woody species in the New Zealand flora, probably because of the very low frequency fire regimes prevalent during New Zealand’s eco-evolutionary history (Perry et al. Citation2014). Fire adaptation is possibly shown in (the restricted range) Pomaderris hamiltonii (Rhamnaceae), which shows smoke- and heat-stimulated germination (Haines et al. Citation2007), and Discaria toumatou (matagouri; Rhamnaceae), which has a ‘double-dormancy’ mechanism that may potentially represent a fire adaptation (Rowarth et al. Citation2007), although this requires experimental testing.
The most obviously fire-adapted species in New Zealand’s woody flora is Leptospermum scoparium J.R. et G.Forst. (mānuka), a shrub to small tree found throughout New Zealand. This member of the Myrtaceae is considered a relatively recent immigrant (likely 2–3 Ma) to New Zealand (Thompson Citation1989; Battersby et al. Citation2017) from Australia, where the species also occurs. In New Zealand, L. scoparium exhibits geographically-variable pyriscent serotiny (Wardle Citation1991; Harris Citation2002; Bond et al. Citation2004; Battersby et al. Citation2017), with both serotinous and non-serotinous individuals in most populations. Harris (Citation2002) and Battersby et al. (Citation2017) describe a strong north–south gradient in the prevalence of serotiny in populations and individuals of Leptospermum scoparium; Battersby et al. interpret this pattern as the outcome of recurrent fire in the north of New Zealand, especially in restiad wetlands, and a lack of vegetation cover during the Last Glacial period in the south. Alternatively, Bond et al. (Citation2004), who considered South Island populations only, found little evidence for a latitudinal effect on serotiny. Presumably, serotinous genotypes are favoured in areas where fire is recurrent and non-serotinous ones are able to exploit other colonisation opportunities in inter-fire periods; Goubitz et al. (Citation2004) describe a similar ‘dual strategy’ in Pinus halapnesis in Israel.
While prolonged exposure to heat is lethal to the seeds of L. scoparium (Burrell Citation1965), its germination seems unaffected following heating within capsules during fire. Burrell (Citation1965) investigated germination rates of L. scoparium seeds from central Otago (South Island, New Zealand) and observed in laboratory trials that heat shocking of capsules by exposure to an air temperature of 100 °C killed all seeds. However, Burrell (Citation1965, p. 5) also comments that ‘seed from capsules that were opening within an hour of the passage of a fire germinated as well as, but no better than, seed from plants that were merely scorched … ’. In Australia, L. scoparium shows strong fire adaptations including pyriscent serotiny and resprouting from lignotubers, the latter of which is not known in New Zealand L. scoparium (Bond et al. Citation2004). In Australia, heating trials suggest that other Leptospermum spp. may show positive germination responses to heating of the capsule (Judd Citation1993), although there was no indication of this being statistically significant and one of the species that Judd (Citation1993) assessed, L. myrsinoides, is not serotinous by the definition of Lamont et al. (Citation1991). Jefferson et al. (Citation2014) record positive germination response to smoke treatment in some Leptopsermum taxa. However, other than Burrell’s (Citation1965) single trial these effects have not been experimentally assessed in New Zealand L. scoparium.
In this article we ask whether the fire adaptations seen in the Australian members of the genus are present in its sole New Zealand representative. If L. scoparium in New Zealand is fire-adapted then we would expect that, in populations where serotiny is prevalent, heating could have a positive, or at least neutral, effect on germination rate and total germination success. Here we make the first systematic evaluation of evidence for heat-stimulated germination of L. scoparium in a germination trial using capsules collected from 12 populations from the far north of the North Island (34.27°S) to the southern South Island (46.33°S). We also assess whether there are associations between population-level serotiny, shoot flammability and seed germination. We ask the following:
Does heat stimulation (short exposure of mature capsules to high temperatures) affect seed germination in L. scoparium and, if so, is this effect consistent across populations?
Does germination success/rate vary with serotiny and/or shoot-level flammability?
Methods
Study taxon
Leptospermum scoparium (mānuka; Myrtaceae) is a shrub to small tree (up to 5 m height) that is found across New Zealand and southeastern Australia (Thompson Citation1989). The species shows genotypic (Ronghua et al. Citation1984) and ecotypic (Lee et al. Citation1983; Price and Morgan Citation2006) plasticity, tolerates a broad range of environmental conditions and is prominent in a wide range of communities (Stephens et al. Citation2005). Leptospermum scoparium occurs most often as even-aged stands in the initial stages of forest succession and on infertile soils (Williams et al. Citation1990; Clarkson et al. Citation2011); it is particularly common in mires below treeline (McGlone Citation2009).
Material collection and storage
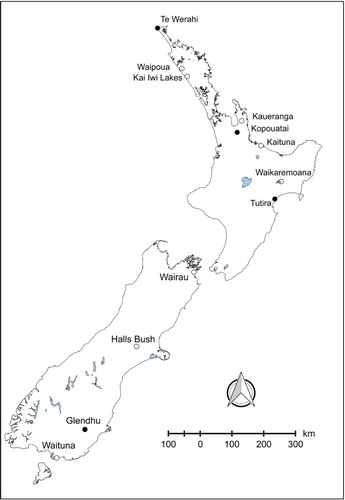
We collected closed capsules from 12 L. scoparium populations along a latitudinal gradient (, Table S1). Published palaeocharcoal records from these sites show that they have all experienced frequent fire since humans arrived in New Zealand c. 750 years ago (Wilmshurst et al. Citation2008), with some also experiencing episodic fire during the late Holocene prior to human arrival (). Capsules were harvested from random locations on branches from randomly-selected individuals to ensure a range of capsule ages was captured, although Mohan et al. (Citation1984a) found no evidence that capsule age does affect germination success. At each site population-level serotiny was assessed as the proportion of individuals with open capsules, following the methods described by Bond et al. (Citation2004) and Battersby et al. (Citation2017).
Figure 1. Location of the 12 sites from where capsules of Leptospermum scoparium were collected. Sites with closed circles have experienced fire since c. 3000 yr BP and those with open circles since human settlement in c. 1280 (Battersby Citation2014). Flammability experiments were conducted on material from all sites except Kai Iwi Lakes.
Forty capsules from each site were removed and divided at random into two groups; one to be heated and one to remain unheated (i.e. a control). Heat-stimulated capsules were stored at 4 °C to inhibit capsule opening, control groups were left at room temperature to open naturally. It was necessary to store the capsules to be heat stimulated in cold conditions to prevent them from opening naturally. However, reproductive populations of L. scoparium are found in much colder conditions (Greer et al. Citation1991) and the foliage damage temperature for the species is also considerably lower than 4 °C (down to −8 °C; Warrington and Stanley Citation1987; Bannister Citation2003). Baylis (Citation1958) observed L. scoparium capsules opening under extreme cold but this did not occur in our case. When control groups started opening, capsules in the heated group were raised to room temperature overnight, heated and left to open. This process took approximately 2 months from sampling to heat stimulation and then approximately another month until germination trials commenced. The 12 populations were sampled over three separate periods resulting in three separate batches of experimental trials.
Heat stimulation and germination protocol
Capsules were heated following the protocol of Judd (Citation1993, Citation1994). Twenty capsules from each site were positioned on wire gauze placed over an aluminium tray (Figure S1). This design ensured that the primary mode of heat transfer was convective rather than conductive, similar to real fire events (Judd Citation1993). The tray was placed in an Elecfurn muffle furnace (Electric Furnace Co, Model 3025 50) preheated to 200 °C, for 15 s, then removed. Judd (Citation1993) demonstrated that exposure to reasonably low temperatures for short durations was optimal for germination in Leptospermum. Burrell (Citation1965) observed exposure to 100 °C for 5 min was lethal to Leptospermum seeds in capsules. We designed our treatment to simulate the passage of a fire through a fuel bed that would open serotinous capsules rather than the type of heating that would stimulate germination from soil seed banks, which are not important in L. scoparium (Mohan et al. Citation1984a). After heating, capsules were removed from the wire gauze and allowed to return to room temperature before being returned to unsealed paper bags and left to open naturally. The other possible heating treatment would be to expose seeds to heat (i.e. outside of the capsule) but: (1) this would almost certainly just kill them; and (2) in the post-fire environment the seed bank for L. scoparium is aerial in capsules rather than soil-stored (Mohan et al. Citation1984b).
Seeds were germinated following the protocol described in Mohan et al. (Citation1984a) on two layers of Whatman No. 1 filter paper moistened with 3 mL of deionised water in 90 mm plastic petri dishes. Five dishes were used per site with c. 100 seeds per dish (c. 500 seeds total per site) which were then separated into heated and control groups. Dishes were placed together on two shelves in a growth chamber (Contherm Industries, Model 620RHS) at 23 °C under a 14 h photoperiod of 35 µE m−2 sec−1 light intensity at 80% humidity and atmospheric CO2. We replaced water as required to keep the paper moist. Mohan et al. (Citation1984a) reported that germination started after 3 days and was finished after 7 days, with few seeds germinating beyond that. Thus, recordings were made daily for at least 15 days (at which point germination had ceased) and radicle emergence was taken as the criterion for germination completion. Because of the logistics of seed collection, experiments were conducted on three separate occasions.
Although a large number of seeds (more than 1 × 104) were used in the trials, Corner (Citation1976) notes that the capsules of some members of the Myrtaceae contain many unfertilised ovules as well as seeds. If this were the case then our viability estimates, although similar to previous studies (Mohan et al. Citation1984a; Sessions and Kelly Citation2000), are potentially under-estimates as the experiment included viable seeds and unfertilised material. Corner (Citation1976) provides no specific information about Leptospermum and minimal information about Kunzea (L. scoparium was formerly K. scoparium).
Flammability assessment
We assessed shoot-level flammability in Leptospermum scoparium using the device designed by Jaureguiberry et al. (Citation2011) and the protocol described in Wyse et al. (Citation2016) and Battersby et al. (Citation2017). The material collected for the flammability experiments was sampled from 11 of the 12 populations from which we also obtained the capsules used in the germination trial (). Flammability was evaluated from two 70 cm branches (including leaves and capsules) from five individuals from each site (so n = 10 per site). Four measures of flammability were quantified—time to ignition (ignitability), the duration of burn (sustainability), maximum burn temperature (°C) (combustibility) and percentage of sample burnt (consumability)—and these were synthesised via principle component analysis (PCA). The first axis of this PCA represents an overall measure of flammability and the second a measure of ignitability for the 11 populations that overlap with seed germination (Figure S2).
Table 1. Summary of general linear mixed models of effects of heat shocking on seed germination (‘germinability’) and rate of germination.
Statistical analysis
We summarised the germination experiments using: (1) the proportion of germinated seeds at the cessation of the trial (15 days); and (2) the number of days after which 50% of those seeds that did germinate on a given petri dish had germinated. The first measure provides an index of overall germination success (‘germinability’; sensu Ranal and de Santana Citation2006) and the second the rate at which germination occurred. We analysed these data using mixed effects logistic and linear regression models with treatment as a fixed factor and population, trial and petri dish as random effects (dish nested in population). We included the random effects because models including just the heat treatment suggested over-dispersal (residual deviance >> degrees of freedom) and models with the (population | dish) term out-performed one that included a population term alone (AICc = 5.0 and 6.4 for germinability and germination rate, respectively). Models were fitted using the lme4 library (Bates et al. Citation2015) using R-3.2.0 (R-Development-Core-Team Citation2016).
Results
Across all experimental trials 1383 of 11,986 seeds germinated, representing an overall final germination success of 11.5%, with a population-level mean of 12.2% (n = 12) ranging between 5.5% and 24.0%. The proportion germinated varied across the three experimental batches (means: 9.8%, 13.5% and 15.1%).
Germinability
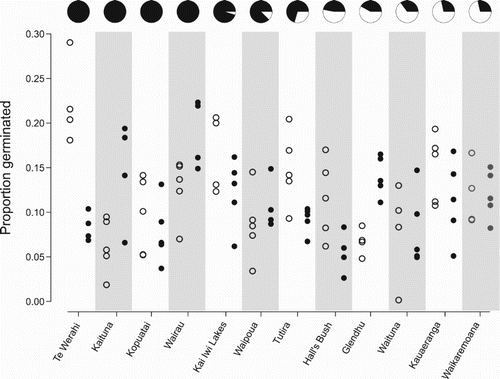
The overall (i.e. pooled over populations) effect of the heat treatment was to lower slightly the final germination success (means: 13.5 ± 7.1 vs 11.5 ± 4.9% [mean ± 1 SD]; A). Across all replicates, the control group, when compared to the heated group, had a larger range of germination success (0%–29% vs 3%–23%). However, a mixed effects logistic regression model showed no significant effect of heat exposure on germination success (β = −0.102 ± 0.059 [SEM], z = 1.731, P = 0.083) and response varied considerably between populations (). The Glendhu population showed the strongest positive response to heat shocking (14% vs 7%). Kaituna, Wairau and Waipoua also showed positive responses to heat treatment. Te Werahi and Hall’s Bush showed the strongest negative effects of heat shocking ().
Figure 2. A, Pooled effect of heating (blue = unheated vs red = heated) on Leptospermum scoparium seeds across all populations; B, effects of heating on germination rate of seeds of L. scoparium. Heavy lines are means and shaded areas ± 1 SD pooled across all populations.
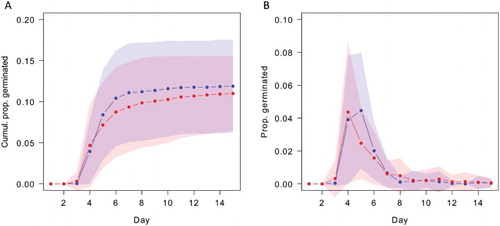
Figure 3. Effects of heating for individual sites. Open dots represent no heat-treatment replicates and closed dots represent heat-treated replicates. Note the high level of variability in germinability both within and between sites. The pie charts above each site show the proportion of serotinous individuals (> 60% closed capsules) at that site (in black), and sites are in descending order of prevalence of serotiny (left to right).
Germination rate
The heating treatment effect slightly slowed germination rate (5.25 vs 5.77 days; pooled medians), but this was not statistically significant and varied little between populations (β = 0.420 ± 0.253, t = 1.665, P = 0.102; ). Of the 12 populations, five (41%) showed a positive response to heat shocking and seven a negative one (59%).
Association with flammability and serotiny
There was no correlation between population-level serotiny and heat treatment (proportional effect of heat relative to control; ρ = 0.039), indicating that serotiny level is not associated with a germination response to heat treatment. Likewise, the two PCA-based flammability measures and response to heat-shocking were uncorrelated (ρ = 0.091 and −0.173 for PCA axes 1 and 2, respectively). Geographic parameters (latitude, elevation) did not predict germinability (ρ = 0.349 and −0.224, respectively; Figure S3).
Discussion
A number of populations of L. scoparium in New Zealand exhibit serotiny and this trait has been interpreted as pyriscent (Wardle Citation1991; Bond et al. Citation2004; Battersby et al. Citation2017). We experimentally evaluated the effect of heat stimulation on the germinability and germination rates of L. scoparium from 12 populations across New Zealand; by extension this is also an evaluation of the insulation properties of the capsules of L. scoparium. To assess whether any heating effect on germination is associated with flammability we also measured shoot-level flammability in 11 of these 12 populations. However, although Burrell (Citation1965) demonstrated that prolonged exposure to heat is lethal to L. scoparium seeds within capsules, our experimental heat treatment elicited no consistent effect of heat stimulation on germinability or germination rate either within or between populations. The effect of heating on germination success varied considerably between populations and was positive in some (5/12) and negative (7/12) in others. Likewise, there was no relationship between either germinability and serotiny, or germinability and flammability. Battersby et al. (Citation2017) and Harris (Citation2002), but not Bond et al. (Citation2004), describe a strong north–south gradient in the prevalence of serotiny in L. scoparium populations; however, geographic parameters (latitude, elevation) did not predict germinability.
Does heat stimulation influence seed germination in Leptospermum scoparium?
Studies of vegetation recovery in New Zealand wetlands have reported abundant L. scoparium seedlings shortly after fire (Timmins Citation1992; Clarkson Citation1997; Johnson Citation2001), suggesting that the species germinates effectively and out-competes other woody seedlings in these conditions. The dynamics described in these studies suggest that L. scoparium capsules effectively insulate seeds during fire and that the seeds remain viable for some period afterwards. Mohan et al. (Citation1984a) note that, because there is little storage of L. scoparium in the soil, the aerial seed bank is crucial for recruitment, making effective insulation during fire crucial. However, in our trials there was no consistent effect of short-term exposure to elevated temperatures on seed germination across L. scoparium populations distributed along a latitudinal gradient across New Zealand. This result is consistent with the observations of Burrell (Citation1965) who showed that Leptospermum seeds from populations in Otago were unaffected, or at least not destroyed, by fire.
Our experiments highlighted a large degree of inter-population variation in the response to heat exposure, with some populations, such as Te Werahi, exhibiting a strong negative response and others, such as Glendhu, showing a strong positive response (). The overall average germination success of 11.5% is comparable to the 13% reported by Mohan et al. (Citation1984a) and the 15% by Sessions and Kelly (Citation2000) under glasshouse conditions. Neither Mohan et al. nor Sessions and Kelly applied a heat treatment to seeds, but the results from our study further confirm that L. scoparium, whether heat stimulated or not, has a low germination rate even in the glasshouse (with the caveat that some of the material in the capsule may not represent viable seed). Peak germination in the heat-stimulated treatment group was slightly earlier than for the control group (B). However, this effect was not statistically significant and the median germination day was the same for the control and treatment. That heat treatment did not consistently negatively affect germinability, suggesting that the capsules provide adequate insulation for the treatment we applied, lends further support to the view that serotiny in L. scoparium is a fire adaptation.
Do germinability and/or germination rate vary with serotiny and/or shoot-level flammability?
No previous studies have simultaneously assessed flammability, serotiny and the effects of heat exposure on seed germination. These traits were not associated in L. scoparium (although they have been predicted to be under frequent fire), suggesting that either the association has disappeared under the low frequency fire regimes experienced by the species in New Zealand over most of its evolutionary history, or the coordination between these traits never existed. Previous studies of coordination among fire traits have focused on species in crown-fire ecosystems; the small stature of L. scoparium means that entire plants are likely consumed in fire and that this is sufficient to trigger seed release. As a result, the need for effective heat transfer to the canopy is reduced and so a positive association between flammability and serotiny is less important.
Leptospermum scoparium fire traits in the context of other (Australian) Leptospermum
Reproductive adaptations to fire are common in other members of Leptospermum. Judd (Citation1993) found some evidence of an increase in germination for serotinous L. myrsinoides after shocking at higher temperatures than for the other Myrtaceae species he tested. Ashton (Citation1986) describes survival of Leptospermum seeds after exposure to a heat of 320 °C for 4 min (considerably more intense than the lethal level for L. scoparium reported by Burrell Citation1965). Again, the key difference here, compared to New Zealand, is the long history of fire in the systems these taxa live in; strong serotiny and the presence of effectively insulating capsules/cones will be favoured under frequent fire regimes.
Leptospermum scoparium exhibits pyriscent serotiny in both Australia and New Zealand, although in New Zealand the strength of serotiny varies within and between populations (Harris Citation2002; Bond et al. Citation2004; Battersby et al. Citation2017). However, while in mainland Australia and Tasmania L. scoparium resprout from lignotubers after fire, this resprouting ability is apparently absent in New Zealand populations (Bond et al. Citation2004). While resprouting is favoured over serotiny under high frequency fire regimes (Enright et al. Citation1998), it also requires the maintenance of stored carbohydrates and nutrients (Moreira et al. Citation2012) and so carries a significant cost. If we assume that resprouting was present when L. scoparium arrived in New Zealand from Australia, then its absence suggests a low frequency fire regime with little selective strength in New Zealand prior to human arrival.
How ‘fire adapted’ is Leptospermum scoparium?
Across its New Zealand range Leptospermum scoparium exhibits no apparent relationship between serotiny and both fire history and flammability (Battersby et al. Citation2017; but cf. Bond et al. Citation2004), no link between germination success rate and serotiny or flammability, and no increase in germinability following brief and low level heat stimulation. All of these findings point to a loss of some key fire adaptations in many New Zealand L. scoparium populations. The fact that heat stimulation does not decrease germinability supports the idea of pyriscent serotiny in the species; but the species’ other characteristics suggest that such adaptation is weak. There is no doubt that L. scoparium can rapidly colonise post-fire environments, but over most of its history in New Zealand it has inhabited ecosystems where fire is rare, alongside a flora with virtually no fire adaptations at all. In such circumstances even weak adaptation to fire may confer a significant competitive advantage in post-fire settings. When L. scoparium co-occurs with pyrophylic invasive taxa such as Ulex europeaus and Hakea spp. it can be over-topped and out-competed in post-fire environments (Perry et al. 2010), showing that, at least on poor soils, other fire-adapted species grow more rapidly. It is worth noting that the taxonomy of L. scoparium and its intra- and inter-specific relationships with Australian Leptospermum requires further consideration (Stephens et al. Citation2005), especially given the evidence of ‘regional divergence’ (Thompson Citation1989, p. 335) and ecotypic and genotypic variability within the species (Lee et al. Citation1983; Ronghua et al. Citation1984). This variation is recognised, for example, in the form of the northern subspecies L. scoparium var. incanum (New Zealand PCN Citation2016)
Conclusions
We found no relationship between seed germination under heat stimulation, and either flammability and serotiny in L. scoparium populations across New Zealand. Heat stimulation, at least at the intensity we used, did not affect germination success, indicating that the capsules of L. scoparium are effective insulators. There was considerable variation in germination response among sites, with some positively affected, some negatively affected and some not affected by heat stimulation of seeds within capsules. Our trials, while broad in geographic scope, are based on a single heat treatment (albeit one informed by knowledge for other Leptospermum). Further studies could usefully assess germination response in Leptospermum across a wider range of heating conditions (temperature and duration) and also whether there is a germination response to smoke. Likewise, it would be informative to further investigate the insulation capacity of L. scoparium capsules (e.g. wall thickness, capsule size and moisture content in relation to capsule age). While in fire-prone ecosystems reproductive traits may be correlated, in New Zealand’s most obviously fire-adapted woody species they are not. This lack of correlation suggests that such traits have either been lost under the low frequency fire regimes experienced by Leptospermum in New Zealand over most of its history, or that they never existed.
Table S1
Download MS Word (42 KB)Figure S1
Download MS Word (675 KB)Figure S2
Download MS Word (305 KB)Figure S3
Download MS Word (92 KB)Acknowledgements
We thank two anonymous referees and Janice Lord for very useful comments on an earlier draft of this manuscript. Thanks to Joan and Juliet Battersby for help with field data collection; Phil Holland and Dean O’Connell who helped with the flammability experiments; Kelly Booth for help with the germination trials; and Dave Wackrow for help with the seed heating treatments. Associate Editor: Dr Janice Lord.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Janet M. Wilmshurst http://orcid.org/0000-0002-4474-8569
George L. W. Perry http://orcid.org/0000-0001-9672-9135
Additional information
Funding
References
- Ashton DH. 1986. Viability of seeds of Eucalyptus obliqua and Leptospermum juniperinum from capsules subjected to a crown fire. Australian Forestry. 49:28–35. doi: 10.1080/00049158.1986.10674460
- Bannister P. 2003. Are frost hardiness ratings useful predictors of frost damage in the field? A test using damage records from the severe frost in South Otago and Southland, New Zealand, July 1996. New Zealand Journal of Botany. 41:555–569. doi: 10.1080/0028825X.2003.9512869
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 67(1):1–48. doi:10.18637/jss.v067.i01
- Battersby PF. 2014. Reproductive ecology of mānuka (Leptospermum scoparium) under various fire regimes [Unpublished MSc Thesis]. School of Environment, University of Auckland.
- Battersby PF, Wilmshurst JM, Curran TJ, McGlone MS, Perry GLW. 2017. Exploring fire adaptation in a land with little fire: serotiny in Leptospermum scoparium (Myrtaceae). Journal of Biogeography. 44:1306–1318. doi: 10.1111/jbi.12950
- Baylis GTS. 1958. An example of winter injury to silver beech at moderate altitude. Proceedings of the New Zealand Ecological Society. 6:21–22.
- Bond WJ, Dickinson KJM, Mark AF. 2004. What limits the spread of fire-dependent vegetation? Evidence from geographic variation of serotiny in a New Zealand shrub. Global Ecology and Biogeography. 13:115–127. doi: 10.1111/j.1466-882X.2004.00070.x
- Bowman D, French B, Prior LD. 2014. Have plants evolved to self-immolate? Frontiers in Plant Science. 5:590. doi:10.3389/fpls.2014.00590
- Burrell J. 1965. Ecology of Leptospermum in Otago. New Zealand Journal of Botany. 3:3–16. doi: 10.1080/0028825X.1965.10428708
- Clarkson BR. 1997. Vegetation recovery following fire in two Waikato peatlands at Whangamarino and Moanatuatua, New Zealand. New Zealand Journal of Botany. 35:167–179. doi: 10.1080/0028825X.1997.10414153
- Clarkson BR, Smale MC, Williams PA, Wiser SK, Buxton RP. 2011. Drainage, soil fertility and fire frequency determine composition and structure of gumland heaths in northern New Zealand. New Zealand Journal of Ecology. 35:96–113.
- Corner EJH. 1976. The seeds of dicotyledons. Cambridge (NY): Cambridge University Press.
- Delgado JA, Serrano JM, López F, Acosta FJ. 2001. Heat shock, mass-dependent germination, and seed yield as related components of fitness in Cistus ladanifer. Environmental and Experimental Botany. 46:11–20. doi: 10.1016/S0098-8472(01)00076-4
- Dwyer JM, Laughlin DC. 2017. Constraints on trait combinations explain climatic drivers of biodiversity: the importance of trait covariance in community assembly. Ecology Letters. 20:872–882. doi: 10.1111/ele.12781
- Enright NJ, Marsula R, Lamont BB, Wissel C. 1998. The ecological significance of canopy seed storage in fire-prone environments: a model for resprouting shrubs. Journal of Ecology. 86:960–973. doi: 10.1046/j.1365-2745.1998.00311.x
- Goubitz S, Nathan R, Roitemberg R, Shmida A, Ne’eman G. 2004. Canopy seed bank structure in relation to: fire, tree size and density. Plant Ecology. 173:191–201. doi: 10.1023/B:VEGE.0000029324.40801.74
- Greer DH, Muir LA, Harris W. 1991. Seasonal frost hardiness in Leptospermum scoparium seedlings from diverse sites throughout New Zealand. New Zealand Journal of Botany. 29:207–212. doi: 10.1080/0028825X.1991.10416722
- Haines L, Ennis IL, Blanchon DJ, Triggs CM. 2007. Propagating the pale-flowered kumarahou (Pomaderris hamiltonii) and kumarahou (Pomaderris kumeraho) from seeds. New Zealand Journal of Botany. 45:91–100. doi: 10.1080/00288250709509706
- Harris W. 2002. Variation of inherent seed capsule splitting in populations of Leptospermum scoparium (Myrtaceae) in New Zealand. New Zealand Journal of Botany. 40:405–417. doi: 10.1080/0028825X.2002.9512802
- He T, Lamont BB, Downes KS. 2011. Banksia born to burn. New Phytologist. 191:184–196. doi: 10.1111/j.1469-8137.2011.03663.x
- He T, Pausas JG, Belcher CM, Schwilk DW, Lamont BB. 2012. Fire-adapted traits of Pinus arose in the fiery Cretaceous. New Phytologist. 194:751–759. doi: 10.1111/j.1469-8137.2012.04079.x
- Jaureguiberry P, Bertone G, Diaz S. 2011. Device for the standard measurement of shoot flammability in the field. Austral Ecology. 36:821–829. doi: 10.1111/j.1442-9993.2010.02222.x
- Jefferson LV, Pennacchio M, Havens-Young K, Sollenberger D. 2014. Ecology of plant-derived smoke: its use in seed germination. Oxford (UK): Oxford University Press.
- Johnson PN. 2001. Vegetation recovery after fire on a southern New Zealand peatland. New Zealand Journal of Botany. 39:251–267. doi: 10.1080/0028825X.2001.9512736
- Judd TS. 1993. Seed survival in small myrtaceous capsules subjected to experimental heating. Oecologia. 93:576–581. doi: 10.1007/BF00328968
- Judd TS. 1994. Do small myrtaceous seed-capsules display specialized insulating characteristics which protect seed during fire? Annals of Botany. 73:33–38. doi: 10.1006/anbo.1994.1004
- Keeley JE, Fotheringham CJ. 2000. Role of fire in regeneration from seed. In: Fenner M., editor. Seeds: The ecology of regeneration in plant communities. 2nd ed. Wallingford (UK): CAB-Publishing; p. 311–330
- Keeley JE, Pausas JG, Rundel PW, Bond WJ, Bradstock RA. 2011. Fire as an evolutionary pressure shaping plant traits. Trends in Plant Science. 16:406–411. doi: 10.1016/j.tplants.2011.04.002
- Lamont BB, le Maitre DC, Cowling RM, Enright NJ. 1991. Canopy seed storage in woody plants. Botanical Review. 57:277–317. doi: 10.1007/BF02858770
- Lee WG, Mark AF, Wilson JB. 1983. Ecotypic differentiation in the ultramafic flora of the South Island, New Zealand. New Zealand Journal of Botany. 21:141–156. doi: 10.1080/0028825X.1983.10428538
- McGlone MS. 2009. Postglacial history of New Zealand wetlands and implications for their conservation. New Zealand Journal of Ecology. 33:1–23.
- McGlone MS, Wilmshurst JM. 1999. Dating initial Maori environmental impact in New Zealand. Quaternary International. 59:5–16. doi: 10.1016/S1040-6182(98)00067-6
- McWethy DB, Wilmshurst JM, Whitlock C, Wood JR, McGlone MS. 2014. A high-resolution chronology of rapid forest transitions following Polynesian arrival in New Zealand. PLoS ONE. 9:e111328. doi: 10.1371/journal.pone.0111328
- Mohan E, Mitchell N, Lovell P. 1984a. Environmental factors controlling germination of Leptospermum scoparium (manuka). New Zealand Journal of Botany. 22:95–101. doi: 10.1080/0028825X.1984.10425236
- Mohan E, Mitchell N, Lovell P. 1984b. Seasonal variation in seedfall and germination of Leptospermum scoparium (manuka). New Zealand Journal of Botany. 22:103–108. doi: 10.1080/0028825X.1984.10425237
- Moreira B, Tormo J, Pausas JG. 2012. To resprout or not to resprout: factors driving intraspecific variability in resprouting. Oikos. 121:1577–1584. doi: 10.1111/j.1600-0706.2011.20258.x
- NZPCN. 2016. Leptospermum scoparium var. incanum. Fact Sheet prepared for NZPCN by P.J. de Lange 1 February 2004. Description by P.J. de Lange. http://www.nzpcn.org.nz/flora_details.aspx?ID=2186
- Ogden J, Basher LR, McGlone MS. 1998. Fire, forest regeneration and links with early human habitation: evidence from New Zealand. Annals of Botany. 81:687–696. doi: 10.1006/anbo.1998.0637
- Pausas JG, Moreira B. 2012. Flammability as a biological concept. New Phytologist. 194:610–613. doi: 10.1111/j.1469-8137.2012.04132.x
- Perry GLW, Wilmshurst JM, McGlone MS. 2014. Ecology and long-term history of fire in New Zealand. New Zealand Journal of Ecology. 38:157–176.
- Price JN, Morgan JW. 2006. Variability in plant fitness influences range expansion of Leptospermum scoparium. Ecography. 29:623–631. doi: 10.1111/j.0906-7590.2006.04645.x
- Ranal MA, de Santana DG. 2006. How and why to measure the germination process? Brazilian Journal of Botany. 29:1–11. doi: 10.1590/S0100-84042006000100002
- R-Development-Core-Team. 2016. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing.
- Richardson SJ, Laughlin DC, Lawes MJ, Holdaway RJ, Wilmshurst JM, Wright M, Curran TJ, Bellingham PJ, McGlone MS. 2015. Functional and environmental determinants of bark thickness in fire-free temperate rain forest communities. American Journal of Botany. 102:1590–1598. doi: 10.3732/ajb.1500157
- Ronghua Y, Mark AF, Wilson JB. 1984. Aspects of the ecology of the indigenous shrub Leptospermum scoparium (Myrtaceae) in New Zealand. New Zealand Journal of Botany. 22:483–507. doi: 10.1080/0028825X.1984.10425282
- Rowarth JS, Hampton JG, Hill MJ. 2007. New Zealand native seed germination requirements: a review. New Zealand Journal of Botany. 45:485–501. doi: 10.1080/00288250709509732
- Schwilk DW, Ackerly DD. 2001. Flammability and serotiny as strategies: correlated evolution in pines. Oikos. 94:326–336. doi: 10.1034/j.1600-0706.2001.940213.x
- Sessions LA, Kelly D. 2000. The effects of browntop (Agrostis capillaris) dominance after fire on native shrub germination and survival. New Zealand Natural Sciences. 25:1–9.
- Stephens JMC, Molan PC, Clarkson BD. 2005. A review of Leptospermum scoparium (Myrtaceae) in New Zealand. New Zealand Journal of Botany. 43:431–449. doi: 10.1080/0028825X.2005.9512966
- Thompson J. 1989. A revision of the genus Leptospermum (Myrtaceae). Telopea. 3:301–449. doi: 10.7751/telopea19894902
- Timmins SM. 1992. Wetland vegetation recovery after fire: Eweburn Bog, Te Anau, New Zealand. New Zealand Journal of Botany. 30:383–399. doi: 10.1080/0028825X.1992.10412918
- Wardle P. 1991. Vegetation of New Zealand. Cambridge (UK): Cambridge University Press.
- Warrington IJ, Stanley CJ. 1987. Seasonal frost tolerance of some ornamental, indigenous New Zealand plant species in the genera Astelia, Dicksonia, Leptospermum, Metrosideros, Phormium, Pittosporum, and Sophora. New Zealand Journal of Experimental Agriculture. 15:357–365. doi: 10.1080/03015521.1987.10425582
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 33:125–159. doi: 10.1146/annurev.ecolsys.33.010802.150452
- Williams PA, Courtney S, Glenny D, Hall G, Mew G. 1990. Pakihi and surrounding vegetation in North Westland, South Island. Journal of the Royal Society of New Zealand. 20:179–203. doi: 10.1080/03036758.1990.10426724
- Wilmshurst JM, Anderson AJ, Higham TGF, Worthy TH. 2008. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proceedings of the National Academy of Sciences. 105:7676–7680. doi: 10.1073/pnas.0801507105
- Wyse SV, Perry GLW, O’Connell DM, Holland PS, Wright MJ, Hosted CL, Whitelock SL, Geary IJ, Maurin KL, Curran TJ. 2016. A quantitative assessment of shoot flammability for 60 tree and shrub species supports rankings based on expert opinion. International Journal of Wildland Fire. 25:466–477.
