Abstract
Environmental contamination from mines producing acid rock drainage, which is caused by sulphide mineral oxidation, represents one of the most significant environmental problems facing the international mining industry. This work investigates the mineral morphological effects on the rate of pyrite oxidation and the influence of relict morphological features on rapid oxidation and thus acid generation rates. Laboratory-based kinetic tests were performed on potentially-acid forming rock by measuring changes in pyrite mineralogical compositions, metal release and acid generation over time. The rate of pyrite oxidation is strongly dependent on the reactivity of two pyrite morphological forms (euhedral and framboidal). After 210 days 70–100% of all framboidal pyrite had undergone complete oxidation, which contributed to an initial high acid generation rate (peak concentration of 2927 mg L−1 CaCO3 after 120 days); subsequent acid generation rates (1730 mgL−1 CaCO3 after 390 days) were substantially lower. Scanning Electron Microscopy (SEM) micrographs clearly show the persistence of larger euhedral pyrite grains as a contributing factor to this on-going acidity after 390 days. Samples collected from laboratory humidity cells after 390, 480 and 720 days showed evidence of preferential dissolution associated with these large pyritic overgrowth textures. Clearly evident are prior relict framboid networks within larger euhedral pyrite grains suggesting that oxidative dissolution may be related to internal crystallographic defects associated with the overgrowth textures in these samples.
Introduction
Open cast mining of coal has been undertaken at the Stockton Mine on the West Coast of New Zealand since 1944. The current operator of the Stockton Mine, Solid Energy New Zealand Limited recognises that acidic drainage from waste rock dumps and historic underground mining legacies has led to depressed pH and elevated concentrations of some trace metals in streams draining the Stockton Plateau. Understanding of the potential for acid rock drainage (ARD) from different lithologies on site provides essential information for managing ARD through prevention, minimisation, and treatment strategies.
Economic coal seams within the Buller coalfield are associated with the Brunner Coal Measures (BCM), which are a succession of sedimentary rocks consisting of clastic rocks and coal seams. The BCM unconformably overlie the basement rocks, which are comprised of Greenland Group turbidite sequences, intruded by granites and porphyries. The BCM are Eocene in age and are dominated by sandstones inter-layered with siltstones, mudstones, carbonaceous mudstones, and coal seams. The BCM sequence shown in is 70–130 m thick and was deposited in a fluvioparalic environment consisting of braided and meandering streams within a deltaic, barrier, tidal environment (Flores & Sykes Citation1996). The BCM are conformably overlain by, and laterally interfingered with, the Eocene marine Kaiata mudstone formation (Flores & Sykes Citation1996). In general both the mudstones and sandstones are dominated by quartz, muscovite, and kaolinite with varying minor proportions of microcline, albite, illite, and pyrite; although as expected the sandstones are elevated in quartz compared to the other minerals. The sulphur content and thus pyrite content of rock varies significantly on a geographic basis within the coal mining area and is also constrained lithologically. This is related to the palaeoenvironmental setting. Coarse grained sandstones tend to be low in pyrite and thus low acid forming (LAF), which is due to either deposition in a fluvial (low S) environment or low organic matter availability to convert SO4 2− to H2S (and favour pyrite formation). Mudstones and similar lithologies such as carbonaceous mudstones and siltstones tend to be elevated in pyrite which is linked to deposition in a low energy estuarine environment with good sources of Fe, SO4 2−, and organic matter. This lithological variation in sulphur is shown in , however, geographic variations can affect this assumption. At the southern end of the Stockton Coal Mine within the Mt Frederick mining block the palaeoenvironment was fluvial dominated and thus even organic-rich mudstones can be LAF.
Fig. 1 Generalised stratigraphic column for the Stockton area, West Coast, New Zealand. The circled area is the area of interest, representing the lithological units present at Stockton Coal Mine.
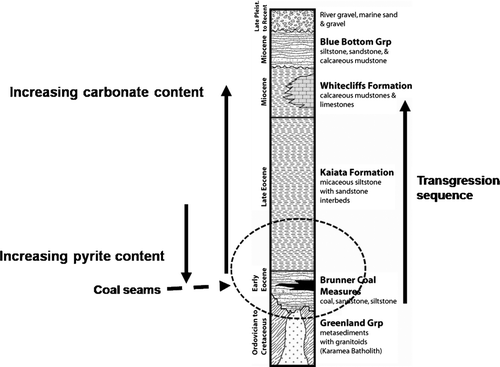
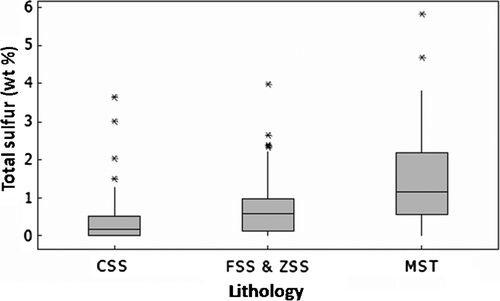
Fig. 2 Distribution of average total sulphur content in lithological units from the Brunner Coal Measures. CSS-coarse sandstones, FSS and ZSS- fine sandstones and siltstones, MST-mudstones. The upper whisker shows the highest data value within the upper limit. The top the box of represents the third quartile. The median is the middle of the data and is shown by the line within the shaded box. The bottom of the box represents the 1st quartile. Asterisks denote outliers, the number of S analyses used to make this figure is >100.
The mechanisms of pyrite oxidation (i.e. kinetics and metal release) have an important role in geochemistry and environmental chemistry of acid rock drainage. Pyrite oxidation and the factors affecting reaction kinetics (oxidative dissolution (O2, Fe3+), temperature, pH, Eh and the presence or absence of microorganisms) have been the focus of extensive study due to their importance in environmental remediation (Nordstrom Citation1982; Weirsma & Rimstidt Citation1984; Mckibben & Barnes Citation1986; Luther Citation1987; Moses et al. Citation1987; Williamson & Rimstidt Citation1994; Evangelou & Zhang Citation1995; Nesbitt & Muir Citation1998; Weisener & Gerson Citation2000a ,Citationb). The oxidation of pyrite will occur when this mineral surface is exposed to either air or water in oxygenated and/or anoxic environments depending on the oxidant, and can involve multiple factors including chemical, biological and electrochemical oxidative reactions. The degree of oxidation and acid generation kinetics can be controlled in part by the morphology, surface area of the individual pyrite, and the degree of armourisation of exposed pyrite in mine spoils (Weber et al. Citation2004; Vaughan Citation2006).
Humidity cells have been widely used to estimate the rates of weathering in order to predict the rates of acid generation from pyrite oxidation. It is generally acknowledged that humidity cells can overestimate reaction rates in the field by up to 3 orders of magnitude but are nevertheless an important and standardised step to understand reaction kinetics. In most geochemical studies kinetic data is often normalised to mass per unit time per unit area. However, it is difficult to accurately measure the reactive surface area of minerals within a mine waste sample, hence other ways of expressing the rates of weathering are now commonly employed that relate the rate to unit sample mass rather than surface area. Typically it is the pyrite oxidation rate that is the critical weathering rate of importance in these types of mine waste studies. Framboidal pyrite introduces another factor when addressing pyrite reactivity as it is prone to considerable oxidation generating significant acidity compared to euhedral pyrite forms (Weber et al. Citation2004).
Framboidal pyrite is common in modern and ancient sedimentary rocks (Schoonen Citation2004; Ohfuji et al. 2005) and are spherical structures consisting of densely packed pyrite crystals with diameters ranging from 1–30 µm (Wilkin et al. Citation1996). Framboids form from the rapid nucleation associated with strongly supersaturated solution through the reaction of aqueous FeS complexes with H2S (Butler & Rickard Citation2000; Rickard & Luthor Citation2007). Since framboids can form within the water column under euxinic conditions or during early digenesis within the top few centimetres of the sediment, their sizes will reflect the redox environmental condition when they were deposited (Wilkin et al. Citation1996). Ohfuji et al. (Citation2005) were able to distinguish morphologically ordered and disordered framboids and determined the individual nanocrystal orientations. Their results suggested that self organised structures result from the continued aggregation and subsequent reorientation of equimorphic nanocyrstals. In coal systems pyrite polyframboids will serve as further nucleation surfaces for secondary overgrowth of pyrite. Overgrowth textures of framboids lead to the formation of homogenous spheres and eventually euhedral pyrite shells (Ostwald & England Citation1979; Wilkin et al. Citation1996).
No studies thus far have investigated the reactive nature (i.e. susceptibility to oxidants) in these types of overgrowth structures. This has significance for understanding how ARD develops from such substrates and can provide valuable information with regards to prevention, minimisation, and treatment options. The focus of this study was to determine the effects of pyrite morphology on acid generation rates using a modified humidity cell and a sample of BCM mudstone from the Stockton Coal Mine.
Materials and methods
Samples
Representative mudstone was used from the Stockton mine site, which was sourced from run-off mine overburden that had been used to construct a 500 tonne weathering trial (Egypt Pad 11). The mineralogy of this sample is dominated by quartz, muscovite and kaolinite with varying minor proportions of microcline, albite, illite and contrasting pyrite morphologies. The sulphur content was 1.67±0.5 wt%. Approximately 1 kg of rock material was crushed and sieved to 53–200 µm for the kinetic humidity cell leach test. Samples from the kinetic humidity cell leach test were collected over 720 days and were sent to Vancouver Petrographics, Vancouver BC, Canada for polished thin section preparation. Sections were studied using a field emission environmental secondary electron microscope (FE-ESEM). All crushed rock samples were collected prior to the washing procedure used to evaluate cell leachate chemistry.
Humidity cells
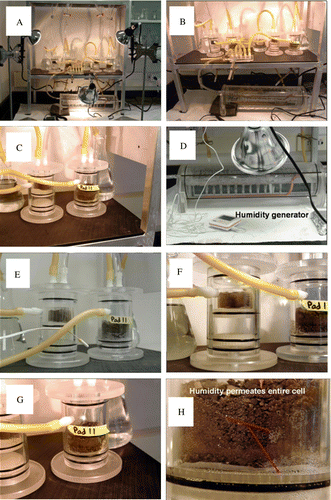
Humidity cell apparatus and procedures were based on a modified version of protocols given in D5744-96 American Society for Testing and Materials (ASTM) (Citation1996) and after White & Lappakko (Citation2000). In this case humidity cells were operated for 720 days to simulate conservative weathering (at 95% humidity) on the BCM mudstone sample (). One humidity cell was used to study the surface corrosion products associated with polished sections of the BCM mudstone material. In this humidity cell, three polished sections were exposed for 210 days to observe initial oxidation of the polished surface. The slides were removed periodically from the humidity cell for FE-ESEM characterisation over this period. The FE-ESEM can operate using low vacuum mode (1 atm) and low primary accelerating currents which eliminate instrumental charging and need for a conductive carbon coating commonly used in high vacuum instruments associated. The other humidity cell was used to simulate weathering effects on the sample of crushed rock. Each humidity cell is 50 mm in diameter and 100 mm in height and 60 g (dry weight) of crushed rock was loaded onto a 22 µm polypropylene mesh that sat on top of a perforated bottom flange within the humidity cell. During the course of the weathering experiments the degree of humidity and temperature (nominally 24.5°C±0.5oC) was cycled to simulate day/night conditions using low temperature heat lamps over a 24 hr period. The cycle consisted of 12 hr of light (i.e. heat c. 26°C; 95% relative humidity) followed by 12 hr with heat lamps turned off (i.e. heat c. 23°C; 85% relative humidity) over the entire 24 month period. The ground sample was rinsed using 50 ml of deionised water at 14, 30, 60, 120, 210, and 390 days. The deionised rinse water was in contact with the sample for 1 hr before draining to a sample collection vial. Additional solid samples were collected at 480 and 720 days although no rinse samples were collected at these times.
Fig. 3 Humidity cell design for the experiments performed on the sample mudstone. (A–D) show the experimental apparatus and humidity generator consisting of a series of heat lamps and forced air bubbling apparatus (D). (E–G) show a close-up view of the humidity cells containing the planar slides and crushed rock material that was exposed to the high humidity condition. (H) shows moisture permeating the entire cell.
SEM characterisation
Non-rinsed rock samples were collected at the specified time intervals from the humidity cell and prepared as polished thin sections for characterisation by Secondary Electron Microscopy (SEM). This was done in order to investigate potential oxidation and secondary mineral associations. The rock samples were examined using a FEI Quanta 200F, field emission environmental secondary electron microscope (FE-ESEM) under low vacuum at 15 kV. The mineral grains were examined using backscattered electron (BSE) and secondary electron (SE) detectors. Energy dispersive spectroscopy (EDS) analysis was performed in order to examine any changes in elemental composition of the pyrite and associated secondary mineralisation over time. Solid samples were collected at 30, 60, 120, 210, 390, 480, and 720 days. Each polished thin section was characterised to monitor changes in the pyrite morphology and investigate the formation of secondary mineralisation associated with the two pyrite morphologies. The EDAX Genesis Particle cluster analysis software version 5.21 was used for modal distribution determinations (EDAX, Mahwah, NJ, USA). This software was used to evaluate both the polished sections and crushed rock sample during the experiment and to measure the proportions of altered or dissolved material associated with the pyrite grain morphology.
Leachate analysis
Both humidity cells were rinsed with 50 ml of doubly distilled ultra pure water for a one hr period. However, only leachate from the humidity cell containing the crushed BCM mudstone sample was characterised. Leachate samples from the humidity cell containing the crushed size fractions were collected over a 13 month period with sampling at 14, 30, 60, 120, 310, and 390 days. Upon collection and measurement of the leachate volume, the pH and Eh were measured using Ethanol-sterilised semi-micro electrodes to monitor changes in pH (Thermo Ross Sure-flow semi micro pH probe) and Eh (Thermo Ross Sure Flow combo redox/ORP). Net acidity was calculated using procedures described by Kirby & Cravotta III (Citation2005) expressed as mgL−1 CaCO3. Samples were filtered through a 0.20 µm nylon syringe filter and saved for determination of aqueous metal concentration. The solutions were acidified using HNO3, and stored at 4° C until analysis. Aqueous metal concentrations (As, Zn, Cd, Ni, Fe, Al, Si, Mg, Mn, Na, S as sulphate) were analysed by inductively-coupled plasma optical emission spectroscopy (ICP-OES).
Results
pH, acidity and metal release from leaching test
A summary of the metal leachate chemistry, pH and acidity production for the humidity cell over the 390 day period is reported in . The leachate chemistry showed a significant decrease in pH from 5.9 to 2.7 after 30 days reaction followed by constant low pH values (). The sample generated the greatest acidity during the first 120 days with peak concentrations reaching 2926 (mg L−1 CaCO3) followed by a subsequent decline to 1730 (mg L−1 CaCO3) after 390 days.
Table 1 Leachate analysis for the BCM crushed rock sample (390 days)
Mineralogical characterisation of the BCM mudstone prior to humidity cell testing
FE-ESEM was used to characterise polished thin sections of the BCM mudstone rock samples prior to the start of the experiment. Pyrite morphology consisted of both course euhedral and framboidal grains totalling a combined 97% of sulphide minerals present with some accessory marcasite. This assessment showed no prior oxidation of the pyrite minerals. Individual pyrite framboid clusters and euhedral pyrite grains were counted and classified into respective size fractions and compared based on the number of framboid clusters vs. course individual grains for the same size fraction. Each framboid cluster was composed of three distinct group size ranges consisting of 100 nm2–5 µm2, 5–10 µm2 and 10–25 µm2. Overall the pyrite consisted of 71% euhedral pyrite and 29% framboid pyrite grains.
Weathering of exposed polished thin sections
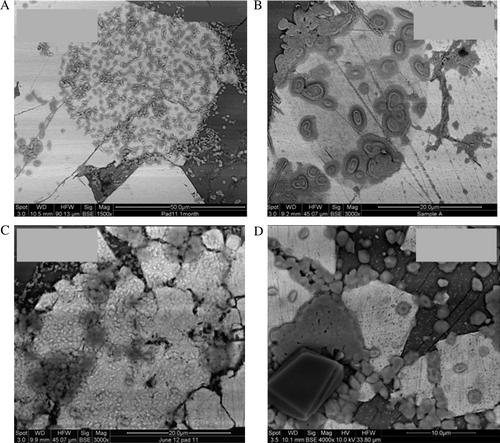
Representative polished thin sections of BCM material (without a cover slip) were placed in one humidity cell for 210 days to study the surface oxidation characteristics of the two pyrite morphologies. Preferential oxidation of the coarse euhedral pyrite showed evidence of corrosion along grain boundaries and specific zones of oxidation were observed on the planar surface within the larger pyrite grains over the period (). These reactive regions represent potential defects and possibly zones of crystallographic weakness associated with either physical and/or chemical defects. After 30 and 60 days in the humidity cell evidence of both chemical and physical alteration show the formation of circular zones resembling relic framboids within some of the larger euhedral grains (a, b). Analysis of these areas using EDS show changes in the pyrite chemical stoichiometry from a 2:1 S:Fe ratio to a 1:1 S:Fe ratio with significant elemental sulphur produced on the surface (b,c, d).
Fig. 4 Successive alteration on the surface of pyrite over a 210 day period was identified from the exposed polished planar slide contained in the humidity cell. (A) After 30 days visible alteration is observed on the pyrite surfaces with the formation of circular zones of secondary precipitation. (B) After 60 days surface alteration products appear to coalesce forming pronounced islands of sulphur; note the diffuse circular region below the secondary mineralised areas resembling ‘relict framboid’ morphology. (C–D), secondary mineralisation continues to form covering the majority of the exposed pyrite.
Weathering of crushed material
Samples of the crushed BCM mudstone were collected from the humidity cells and prepared as polished thin sections to confirm whether preferential oxidation had occurred within the pyrite grains and to test whether the presence of relict framboid textures is a possible pathway for preferential internal oxidation. Distinct differences were observed between the weathering rates of the two principle pyrite morphologies associated with the crushed sample. Framboidal pyrite showed significant alteration within 30 days ranging between 20–40% and after 210 days between 70–100% of all framboid types had undergone replacement (see ). A comparison of the progressive oxidation of both pyrite and framboidal pyrite grains is shown in . After 120 days the samples continued to show evidence of preferential oxidation including the formation of defined circular clusters containing secondary mineralisation products within the euhedral pyrite grains (, ). These latter features appear to be zones of preferential oxidation proximal to overgrowth textures associated with relict framboids. After 120 days between 60–70% of the euhedral pyrite grains within the sample show these alteration patterns. After 210 days between 80–90% of the euhedral pyrite grains show significant alteration and evidence of preferential oxidation of relict framboids. After 390 days all the framboidal pyrite had been replaced with alteration products. In contrast the euhedral pyrite showed significant alteration associated with the preferential removal of the pyrite overgrowth textures evident by the oxidation of relict framboids in the pyrite matrix. After 390 days only 42±5% of the course pyrite grains had undergone alteration, which is significantly less than the framboidal pyrite forms. The element distributions were mapped using the EDAX genesis v5.21 software to determine the chemical spatial relationships within these oxidised areas associated with the euhedral pyrite grains after 390 days. The elemental map in shows the distribution of Fe, K, and Si associated with a large euhedral pyrite grain. Potassium enrichment was associated with the secondary mineralisation suggesting jarosite formation.
Fig. 5 Comparison of progressive oxidation associated with the two pyrite morphologies associated with the crushed rock (not planar slides) contained within the humidity cell after 210 days. Magnifications as shown. (A) shows the percentage alteration observed with the euhedral forms of pyrite and shows successive dissolution textures culminating in c. 40% removal of the perimeter area of iron sulfide. (B) shows the sequential alteration of the framboidal fine grained pyrite culminating in a 90% to 100% alteration or replacement after 210 days.
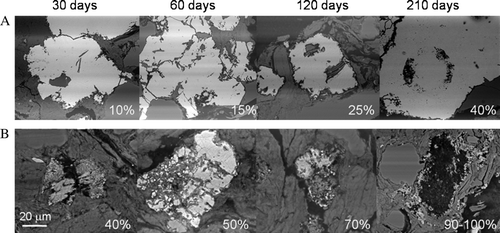
Fig. 6 Elemental distributions from a pyrite grain after 120 day exposure show associated alteration zones. (A) iron (yellow), potassium (red), silicon (blue). (B) Shows the BSE image.
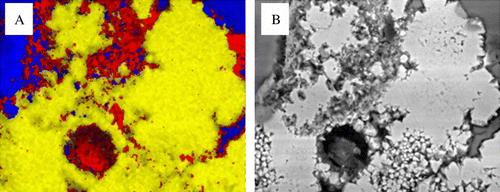
Table 2 Degree of alteration associated with the pyrite morphologies in BCM mudstone sample
Preferential oxidation pathways within rock samples
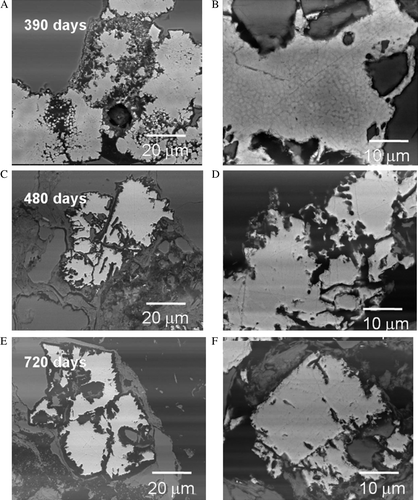
Closer examination of the large euhedral pyrite grains sampled at 390, 480, and 720 days show the development of zones of preferential oxidation which do not appear to result from defined crystallographic orientation. The textures were observed using the BSE detector which shows phase contrast differences between higher Z elements and low Z element concentrations. After 390 days larger euhedral pyrite grains showed evidence of chemical and textural differences and evidence of advanced oxidation. In this case, banding has occurred within the pyrite (). These textures may be a result of preferential oxidation pathways within the pyrite grains via oxygen diffusion. To verify the chemistry associated with the banding within the pyrite, energy dispersive X-ray maps were collected from these pyrite grains (). Darker areas (based on the BSE shown in ) show depletion of high Z elements (such as Fe) and correlates inversely with enhanced oxygen concentration (). It is interesting to note that a comparison of samples collected after 390, 480, and 720 days () show progressive stages of dissolution of the larger euhedral pyrite grains over this time exposure. After 390 days we often observe partial or preferential dissolution of secondary pyritic overgrowth revealing prior framboid cluster development within large euhedral pyrite grains. We also observe evidence of phase contrast differences associated with these areas (). After 480 and 720 days the larger individual pyrite grains in show evidence of dendritic dissolution morphologies within the grains themselves which appear to be reminiscent of the preliminary stages exhibited in and . The rock samples which received longer exposure to weathering appear to be characterised by the dissolution textures along zones of preferential oxidation.
Fig. 7 BSE micrographs collected from prepared thin sections of the crushed material show evidence of zonation within the euhedral pyrite grains after 390 days exposure in a humidity cell. Note different scales. (A) 100 µm, (B) 50 µm, (C) 20 µm, (D) 20 µm.
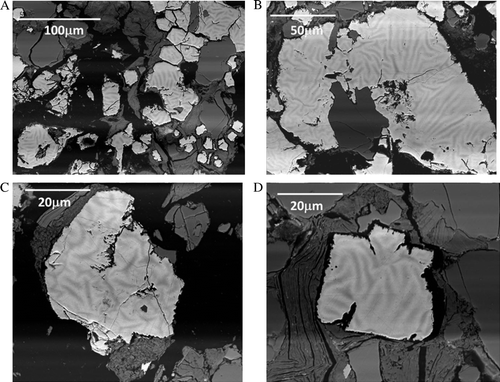
Fig. 8 Chemical maps of zoned pyrite after 390 day exposure. (A) BSE micrograph showing the distribution and phase contrast of low Z elements. (B) Elemental map of oxygen enrichment associated with the dark phase contrast network shown in (A), note the perforated circled area.
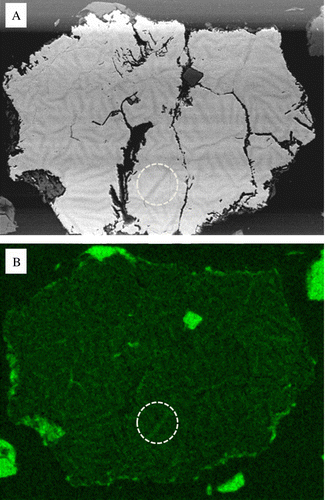
Fig. 9 Comparison of samples showing preferential dissolution of the euhedral pyrite collected after: (A–B) 390 days; (C–D) 480 days; and (E–F) 720 days. After 390 days (top A–B) pyritic overgrowth texture is gradually dissolved showing residual framboid structure within the euhedral pyrite. After 480 days (middle C–D) strong dissolution features are observed within the euhedral pyrite grains. After 720 days (bottom E–F) pyrite grains show continued dissolution along zones of preferential oxidation features similar to those shown in , .
Discussion and conclusions
The oxidation of the sulphide component of waste rock is the critical weathering rate of importance applied to mine waste rock studies (Sapsford et al. Citation2009). Within this context, humidity cells provide an appropriate measure of how rapid acid forming reactions can occur and what limiting steps may influence the overall reaction. This data is especially useful for geochemical model predictions and assessment of possible ARD management and treatment options.
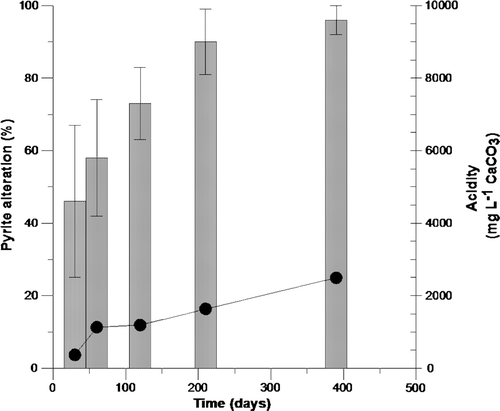
In this study we investigate and compare the reactivity of two morphological forms of pyrite, those consisting of framboid clusters and those comprised of euhedral pyritic grains. Early in the experiments it became apparent that surface area had a significant impact on oxidation rate. A comparison of acidity generated during the experiment with the degree of framboidal pyrite alteration is shown in . A clear correlation is present between the quantities of acid generated as the framboidal pyrite oxidises. Mineralogical investigations indicated that framboid pyrite clusters were prone to greater oxidation compared to euhedral grains and where eventually oxidised within the first 390 days within the humidity cell. Although these forms were oxidised during the experiment, the generation of acidity continued, and was controlled in general, by larger euhedral pyritic assemblage that included internal recrystallised polyframboids. Further study of these grains over 720 days show a sequential oxidation pathway associated with secondary pyritic cements within the large grains. In some instances this results in the oxidation of pyrite from within the mineral in contrast to oxidation of the outer surface. This pathway suggests that oxidation may be occurring chemically along relict framboid grain boundaries and is in some regards a reversible process to original nucleation processes.
Fig. 10 Framboidal pyrite alteration (bar graph) versus cumulative acid release (line graph) for BCM mudstone during the humidity cell experiment.
Ohfuji & Akai (Citation2002) showed that framboid development consist of three dimensional stacking arrangements with a trigonal domain in the centre followed by the progression outwards of tetrahedral domains. This arrangement along with secondary growth of pyrite infilling void spaces may be responsible for facilitating oxidative pathways enhancing the transfer of electrons.
This work clearly demonstrates that one of the dominant drivers for acid generation during the early stages of weathering of the BCM mudstone at the Stockton mine site is the presence of reactive framboidal type pyrite minerals. This is a function of surface area and morphology of the pyrite overgrowth textures. Furthermore, due to crystallographic defects, euhedral overgrowths of internal framboidal cores are also susceptible to accelerated oxidation compared to coarse grained pyrite. In the short term elevated acid generation rates are due to framboidal pyrite oxidation, however, due to their greater modal abundance the long-term acid generation will be from euhedral pyrite oxidation.
This internal reactivity of euhedral and larger massive pyrite grains associated with relict framboidal pyrite has implications for geochemical modelling. Typically for modelling purposes, acid generation rates are based on reaction kinetics (for pyrite) and the available surface area (BET-Brunauer, Emmett and Teller surface area method or geometric); this work demonstrates that internal pathways may also significantly affect oxidation rates and may explain elevated acid generation rates not predicted by models.
Acknowledgements
Thanks to the Staff of Solid Energy New Zealand Limited Stockton mine for help with this project. We also thank staff of GLIER J.C. Barrette for the ICP-OES analysis, Sharon Lackie for the (SEM) analyses.
References
- American Society for Testing and Materials (ASTM) 1996 . D 5744-96 Standard test method for accelerated weathering of solid materials using a modified humidity cell (reapproved 2001) . American Society for Testing and Materials http://www.astm.org/Standards/D5744.htm (accessed 2010) , 13 .
- Blowes , DW and Jambor , JL . 1998 . Modern approaches to ore and environmental mineralogy , 27 Mineralogical Association of Canada .
- Blowes DW , Ptacek CJ , Jambor JL , Weisener CG 2003 . The geochemistry of acid mine drainage Elsevier , 149
- Brown , AD and Jurinak , JJ . 1989 . Pyrite oxidation in aqueous mixtures . Journal of Environmental Quality , 18 : 545 – 550 .
- Butler , B and Rickard , D . 2000 . Framboidal pyrite formation via the oxidation of iron(II) monsulfide byhy drogen sulphide . Geochimica et Cosmochimica Acta , 64 : 2665 – 2672 .
- Evangelou , VP and Zhang , YL . 1995 . A review: pyrite oxidation mechanisms and acid mine drainage prevention . Critical Reviews in Environmental Science and Technology , 25 : 141 – 199 .
- Flores , R and Sykes , R . 1996 . Depositional controls on coal distribution and quality in the Eocene Brunner Coal Measures, Buller Coalfield, South Island, New Zealand . International Journal of Coal Geology , 29 : 291 – 336 .
- Kirby , CS and Cravotta , CA III . 2005 . Net alkalinity and net acidity 1: theoretical considerations . Applied Geochemistry , 20 : 1920 – 1940 .
- Luther , IGW . 1987 . Pyrite oxidation and reduction: molecular orbital theory considerations . Geochimica et Cosmochimica Acta , 51 : 3193 – 3199 .
- Mckibben , MA and Barnes , HL . 1986 . Oxidation of pyrite in low temperature acidic solutions: rate laws and surface textures . Geochimica et Cosmochimica Acta , 50 : 1509 – 1520 .
- Moses , CO , Nordstrom , DK , Herman , JS and Mills , AL . 1987 . Aqueous pyrite oxidation by dissolved oxygen and ferric iron . Geochimica et Cosmochimica Acta , 51 : 1561 – 1571 .
- Nesbitt , HW and Muir , IJ . 1998 . Oxidation states and speciation of secondary products on pyrite and arsenopyrite reacted with mine waste waters and air . Mineralogy and Petrology , 62 : 123 – 144 .
- Nordstrom DK 1982 . Aqueous pyrite oxidation and the consequent formation of secondary iron minerals Acid Sulfate Weathering. Washington, Soil Science Society of American Press .
- Ohfuji , H and Akai , J . 2002 . Icosahedral domain structure of framboidal pyrite . American Mineralogist , 81 : 176 – 180 .
- Ohfuji , H , Boyle , A , Prior , D and Rickard , D . 2005 . Structure of framboidal pyrite: an electron backscatter diffraction study . American Mineralogist , 90 : 1693 – 1704 .
- Ostwald , J and England , BM . 1979 . The relationship between euhedral and framboidal in base-metal sulfide ores . Mineral Magazine , 43 : 297 – 300 .
- Rickard , D and Luthor III , GW . 2007 . Chemistry of iron sulfides . Chemical Reviews , 107 : 514 – 562 .
- Sapsford , DJ , Bowell , RJ , Dey , M and Williams , KP . 2009 . Humidity cell tests for the prediction of acid rock drainage . Minerals Engineering , 22 : 25 – 36 .
- Schoonen , MAA . 2004 . Mechanisms of sedimentary pyrite formation . Geological Society of America Special Paper , 379 : 117 – 134 .
- Weber , PA , Stewart , WA , Skinner , WM , Weisener , CG , Thomas , JE and Smart , RSTC . 2004 . Geochemical effects of oxidation products and framboidal pyrite oxidation in acid mine drainage prediction techniques . Applied Geochemistry , 19 : 687 – 694 .
- Weirsma , CL and Rimstidt , JD . 1984 . Rates of reaction of pyrite and marcasite with ferric iron at pH 2 . Geochimica et Cosmochimica Acta , 48 : 85 – 92 .
- Weisener , CG and Gerson , A . 2000a . An investigation of the Cu(II) adsorption mechanism on pyrite by ARXPS and SIMS . Minerals Engineering , 13 : 1329 – 1340 .
- Weisener , CG and Gerson , A . 2000b . Cu(II) adsorption mechanism on pyrite: an XAFS and XPS study . Surface and Interface Analysis , 30 : 454 – 458 .
- White WW III , Lapakko KA 2000 . Preliminary indications of repeatability and reproducibility of the ASTM 5744-96 kinetic test for drainage pH and sulphate release rate . Proceedings of the 5th International Conference on Acid Rock Drainage. Denver, Colorado . 621 630 .
- Wilkin RT , Barnes HL , Brantley SL 1996 . The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions . Geochimica et Cosmochimica Acta 60 : 3897 3912 .
- Williamson , MA and Rimstidt , JD . 1994 . The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation . Geochimica et Cosmochimica Acta , 58 : 5443 – 5454 .
- Vaughan , DJ . 2006 . Sulfide mineralogy and geochemistry . Reviews in Mineralogy and Geochemistry , 61 : 505 – 600 .