Abstract
The endobenthic ‘watering pot shell’ Clavagella maxwelli n. sp. is described from the Early Miocene, and the similarly endobenthic species Stirpulina cf. pallinupensis Morton, identified hitherto only from the Late Eocene (Priabonian) of Western Australia, is recorded from the late Early Oligocene, both from Otago, New Zealand. Previously, only Clavagella oamarutica Maxwell (Early Miocene) has been recorded from New Zealand. Although the holotypes of Clavagella maxwelli and C. oamarutica have similar tube lengths (c. 80 mm), the two species differ from each other in several important respects. C. maxwelli was a vertically (or nearly so) burrowing endobenthic species whereas C. oamarutica was a cryptic, nestling, possibly cemented species. This is the first record of Stirpulina from New Zealand. The adventitious tube of Clavagella maxwelli was secreted by the mantle from four foci, progressively enclosing the right shell valve and creating an irregular suture on its right side within the array of haphazard tubules that comprises the anterior watering pot.
Introduction
The Clavagelloidea d'Orbigny, 1844 as usually defined (LA Smith Citation1962a; Citationb; Keen & LA Smith Citation1969; BJ Smith Citation1971; Citation1976; Citation1998) comprises a superfamily of sessile, bizarre marine bivalves, usually referred to as ‘watering pot’ shells. Clavagelloidea has recently, however, been shown to comprise two families: Penicillidae Bruguière, 1789 and Clavagellidae d'Orbigny, 1844, both thought to have been derived independently from lyonsiid ancestors, providing a remarkable example of convergent evolution (Morton Citation2007).
In representative genera of the Penicillidae, i.e. Brechites Guettard, 1770, Nipponoclava BJ Smith, Citation1976, Kendrickiana Morton, 2004, Foegia Gray, 1847 and Penicillus Bruguière, 1789 (Morton Citation2002a; Citation2004a; Citationb; Citationc; Citation2006a), both valves of the juvenile shell are incorporated into the fabric of a much larger calcareous adventitious tube or, in the case of the cemented genus Humphreyia Gray, 1858, a crypt, and cannot grow any further (Morton Citation2002b; Citation2007). In representative genera of the Clavagellidae, only the juvenile left valve is incorporated into the structure of either the crypt of endolithic species of Clavagella Lamarck, Citation1818, Dacosta Gray, 1858, Dianadema Morton, 2002 and Bryopa Gray, 1847 or the adventitious tube of Stirpulina Stoliczka, Citation1870 (Owen Citation1835; Morton 1984aMorton Citation2003; Citation2005; Citation2006b; Citation2009). The post-juvenile right valve is free within the tube and can continue to grow (Morton Citation2007). The methods of tube or crypt formation in representatives of the two families are therefore different.
As currently defined (Morton Citation2007), the Clavagellidae includes representatives of the extinct genera Clavagella and Ascaulocardium Pojeta & Sohl, Citation1987 as well as the extant Dacosta, Dianadema, Bryopa and Stirpulina. The first two genera include either boring or crevice-enlarging endolithic species, whereas representatives of the latter three are endobenthic, endolithic and epibenthic inhabitants of soft substrata, respectively. Recent species of Dacosta, Dianadema and Bryopa are comparatively well known (Owen Citation1835; Soliman Citation1971; Appukuttan Citation1974; Savazzi Citation1982a; Citation2000; Morton 1984a, 2003, 2005), whereas Stirpulina ramosa Dunker, 1882 is the only known extant species of tube-dwelling Clavagellidae and is restricted to Japan (Habe Citation1952). Kuroda & Habe (in Kuroda et al. Citation1971: 481) proposed the genus Stirpuliniola for this species (Habe Citation1977: 315, pl. 66, fig. 4), but we consider it to be a synonym of Stirpulina. Nothing is known of either its anatomy or its biology, although Morton (Citation2006b) examined its adventitious tube formation.
According to Savazzi (Citation2000: 323), Clavagella and its allies probably appeared in the Late Cretaceous of the Tethys (LA Smith Citation1962b) and radiated thereafter to become near-cosmopolitan, with early representatives described from the United States, including the Turonian of California (Stallwood Citation1995), Europe, Africa and India (Forbes Citation1846; Stoliczka Citation1870–1871; LA Smith Citation1962b; Savazzi Citation1982b). The only clavagellid recorded from India is the small (c. 12 mm) Clavagella semisulcata (Forbes Citation1846) from the Maastrichtian Valaudayur beds of Pondicherri, southern India (Kossmatt Citation1897; Sundaram et al. Citation2001).
The genus Clavagella became restricted to Europe during the Palaeocene but migrated westwards to Florida during the Eocene (Nicol Citation1968; Pojeta & Sohl Citation1988; Jones & Nicol Citation1989). Pojeta & Sohl (Citation1987) described species from Upper Santonian (Cretaceous) rocks of Mississippi. In the Oligocene, the preserved fossil record suggests that Clavagella extended its range eastwards into the Indo-West Pacific reaching Japan, the Philippines and Australasia in the Miocene. Recent species of the Clavagellidae occur in the Mediterranean (Soliman Citation1971), South Africa (Kilburn Citation1974), Asia and Australia (Morton 1984a, 2005, 2007).
Prior to the discovery of Eocene fossils of Stirpulina in southern Australia (Morton Citation2006c), only one other fossil clavagellid had been recorded from Australia, i.e. Clavagella [as Aspergillum (Humphreyia)] liratum (Tate, Citation1887) (Darragh Citation1970). This species is now assigned to Dianadema, and is possibly a junior synonym of D. multangularis (Tate, Citation1887) (Morton Citation2003). Similarly, only one fossil clavagellid – C. oamarutica Maxwell, Citation1978 – has been described from New Zealand previously, from Altonian (late Early Miocene) rocks (Maxwell Citation1978: 28, figs. 6–9) (). However, a second species of Clavagella is described herein from the Early Miocene of New Zealand. Also, one of us (BM) received two casts of another clavagellid from New Zealand, both virtually indistinguishable from Stirpulina pallinupensis Morton, 2006. Hitherto, S. pallinupensis has been recorded only from the Pallinup Formation (Late Eocene, Bremer Basin) of Western Australia (see Darragh & Kendrick Citation1980; Citation2000 for a review of the associated fauna). This is, therefore, the first record of the genus Stirpulina from New Zealand.
Systematic palaeontology
PHYLUM MOLLUSCA
CLASS BIVALVIA
SUPERFAMILY CLAVAGELLOIDEA d'Orbigny, 1844
Family CLAVAGELLIDAE d'Orbigny, 1844
Genus Clavagella Lamarck, Citation1818
Clavagella Lamarck Citation1818: 430. Type species (by subsequent designation, Children Citation1823): Clavagella echinata Lamarck, Citation1818, Middle–Upper Eocene, Paris Basin.
Diagnosis
Left valve incorporated into the fabric of a secondarily secreted adventitious tube or crypt; right valve free inside it. Both adductor muscles are present and large. ‘Watering pot’ tubules around the anterior distributed evenly but haphazardly over the entire swollen visceral region of the adventitious tube, rather than anteriormost only, and separated from the visceral region by a marked constriction as in Stirpulina.
Clavagella maxwelli n. sp. A–D, A–I, .
Figure 2 Clavagella maxwelli n. sp., Mount Harris Formation (Altonian, early Miocene), Awamoa Beach, North Otago. (A–C) Holotype, OU44540; A, left side showing location of fused left valve; B, right side showing sutures; C, left valve. D, Paratype, OU44541, left valve.
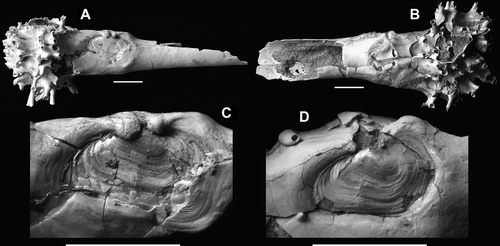
Figure 3 Clavagella maxwelli n. sp., Mount Harris Formation (Altonian, early Miocene), Awamoa Beach, North Otago. (A–D) Paratype, OU44541; A, left side showing fused left valve; B, right side; C, anterior view showing attachment scar of bryozoan fragment; D, enlargement of branching tube on ventral side of crypt. (E–I) Paratype, OU44542; E, anterior view; F, dorsal view of hinge of free right valve; G, external surface of free right valve; H, damaged dorsal side with free right valve as found; I, ventral view.
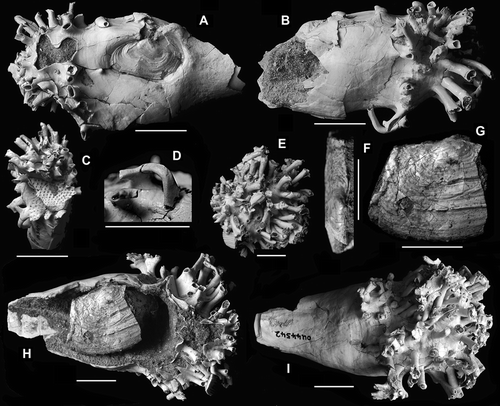
Figure 4 A, Clavagella maxwelli n. sp., holotype, drawing of right side of adventitious tube showing sutures as thick black lines (infilling sediment is hatched). B, Interpretation of how sutures represent eventual unification of components of adventitious tube secreted by four regions (1–4) of the underlying mantle. Orientation and size of left shell valve, incorporated into structure of tube, shown by hatched area.
Type material
Holotype (A–C, ) OU44540, Department of Geology, University of Otago, New Zealand; collected by A Grebneff, 7 August 2005. Two paratypes described below (OU44541, D, A–D; OU44542, E–I). All specimens known so far have been collected from the soft, grey, silty, fine sand of Mount Harris Formation, Altonian local stage (Burdigalian, Early Miocene) at Awamoa Beach, between Oamaru and Kakanui, northeast Otago, South Island, New Zealand (45°O8′N, 170°56′E).
Description
The holotype is slightly flattened along the dorsoventral plane, coinciding with the bedding of the sediment. This has resulted in the fracturing of the tube and tubules. The specimen comprises the watering pot and an adventitious tube that is 80mm long and c. 30mm in dorsoventral diameter (35mm including tubule stumps), with 39mm of tube posterior to the left valve. The right side of the tube is partly missing, exposing the fine grey sand infilling. The diameter of the posterior region of the tube is 14 mm dorsoventrally and 9mm laterally, indicating an undeformed original diameter of c. 11–12mm. The tubules arise from the swollen anterior base of the tube, which narrows abruptly posterior to them and, again, posterior to the left valve, which is incorporated into the structure of the tube. The tubules, up to 11mm long, are haphazardly arranged, most single but some dividing dichotomously. Small, roughened patches among the tubule bases show where fragments of debris were originally cemented to the tube.
The visible part of the left valve, the margins having been overgrown by adventitious tube material in the process of its secretion, measures 13mm in length. Approximately 50% of the outer prismatic layer of the valve has been eroded away, revealing the nacreous internal layer. The remaining outer valve surface shows none of the minute, radially arranged granules typical of many anomalodesmatan shells, including clavagellids and penicillids (Morton Citation2007).
Fine, well-spaced, indistinct radial riblets mark the posterior area of the valve. The valve also displays fine, longitudinal, non-commarginal, anastomosing wrinkles similar to those sometimes seen in Mya truncata Linnaeus, 1758. These wrinkles extend slightly beyond the anterior and posteroventral valve margins and behind the umbo, onto the tube itself, and thus represent an imprint of the periostracum. Both ends of the valve were rounded at mid-growth, but became slightly squared-off before fusion with the tube. The anterior end of the valve is depressed relative to the tube surface. The umbo has been overgrown by two fingers of tube material. From between the fingers a suture extends approximately vertically down the right side of the tube to the mid-point of its dorsoventral height and joins an axial suture extending forwards from a point directly opposite the posterior end of the left valve, directly anteriorwards and weaving discontinuously among the crown of watering pot tubules to their centre. Growth lines on either side of the sutures confirm that they are real structures and not merely a deep fold in the tube wall. A broken-off tubule base forms the posterior margin of the ventral end of the vertical suture opposite the left valve. Another smaller tubule occurs on the dorsal margin of the axial suture just posterior of the watering pot, and is partially embraced by a crushed tubule that has grown from within the suture.
Inclusions of fine sand grain clasts within the tube indicate that sediment entered it during life. The posterior-most remaining part of the tube bears bryozoan etchings and small phoronid boring. If these borings occurred when the specimen was alive, this would indicate that the tube was buried to a depth of only 10mm above the valves.
Paratype 1 (D, A–D), OU44541 (collected by A. Grebneff, 7 August 2005) comprises a 46mm long part of the anterior end of the adventitious tube with tubules and left shell valve incorporated into its structure. Tube diameter is 22mm although it is 29mm across the tubules, the difference being largely due to diagonal crushing along the tube axis. Narrowing of the tube is evident posterior to the tubules and, again, posterior to the left valve. The tubules are almost all missing their tips, except for one intact ventral dichotomous branch which loops posteriorly and then dorsally with a further dichotomous branching at its tip. Large marks indicate that clasts of the heart-urchin Pericosmus crawfordi (Hutton, 1873) (Echinodermata: Echinoidea: Spatangoida) and bryozoan fragments were originally cemented to the tube. The left shell valve is hiatelliform with a down-turned, narrowly rounded, posterior margin and a high, up-turned anterior one. As with the holotype, the valve bears no granules but does display fine, longitudinal, non-commarginal, anastomosing wrinkles, representing the periostracum, which extend onto the tube from the anterior and posteroventral margins of the valve. Radial riblets are not visible due to loss of the outer shell layer posteriorly. The valve margins appear not to have been overgrown by the tube as it was secreted and are set into a narrow depression in the tube surface, beyond which the wrinkles do not extend. Two damaged fingers of tube material embrace the umbo and from between them arises a suture, partially hidden by an out-of-place fragment of tubule-bearing tube, which crosses to the right side of the adventitious tube, there joining an axial suture, which runs forward past one lone tubule that has been folded over it by sediment compaction. The anterior end of the axial suture finishes here due to complete fusion of the components of secreted tube, as will be described. A short (c. 7 mm) section of suture occurring ventrally, but now located right ventrally due to distortion, may be the result of mantle injury during growth. This runs directly under the tip of the prominent recurved tubule. The yellowed (weathered) matrix within the tube has been partially excavated but we could not expose the free right valve. This is either because the valve is flat against one side of the tube, which is impossible to clean out further, or the tube was broken before burial and the valve lost. Paratype 2 (E–I), OU44542 (collected by A Grebneff, 17 May 2003) comprises the ventral, anterior, half (approximately) of an adventitious tube 60 mm long and c. 35 mm wide anteriorly, i.e. where the watering pot of tubules is situated. The tubules arise from the swollen anterior base of the tube that narrows and is broken posteriorly; the broken tube edge reveals a nacreous inner layer. The tubules are haphazardly arranged, some single, others dividing, approximately dichotomously and up to 10 mm long. The left valve formed part of the tube that broke away before burial and so is lost. Internally, and removed from the tube, is approximately two-thirds of the right shell valve (G). The surviving part of the valve comprises the umbo and the posterior region, which appears thickened at the posterior margin but which also gapes. Shell height is 18 mm and the estimated length is slightly greater than 25mm. The prismatic outer shell layer is eroded umbonally to reveal the internal nacreous layer. The large scar (c. 7mm in diameter) of the posterior adductor muscle is located postero-dorsally, above the mid-dorsoventral axis of the valve.
Etymology
The specific name honours the late palaeomalacologist Dr Phillip Alan Maxwell of Timaru, the first to describe a clavagellid from New Zealand.
Distribution, habitat and habits
Clavagella maxwelli n. sp. is known only from the type locality. The species appears to have been endobenthic. The fossils were found lying along the bedding plane, i.e. horizontally. However, they were more likely buried vertically (or approximately so) in life in an outcrop of sandy/silty sediments now located in the infralittoral and normally covered by beach sand and gravel, so that it is uncovered only rarely. The outcrop occurs along the southern 2 km of the 5 km long Awamoa Beach from the junction of Gardiner's Road and Beach Road at the south end to within 200 m of the mouth of Awamoa Creek to the north. The inferred original depth is inner to mid-continental shelf (<200 m). The sediment comprises unbedded, bioturbated, but uniform, medium-grey silt with a fine sand component. No burrows are visible in fresh outcrops but patches of yellowed oxidised surface outcrop are visibly bioturbated, with myriad burrows and faecal strings. That deposition was below the original wave-base is demonstrated by the co-occurrence of large articulated shells of Cucullaea, Lima, Pododesmus and large Pinna (Bivalvia), the latter in life position, and intact crabs and hermit crabs. The presence of a solitary cluster of three adult specimens of the nautiloid Aturia cubaensis (Lea, 1841) indicates that depositional conditions were probably low-energy. The outcrop is soft and eroded to match the normal profile of a sandy beach; when exposed it can be either smooth or cut into rills reminiscent of karstic rillekarren. In contrast, Clavagella oamarutica is believed to have either burrowed in soft sandy/silty sediments or, more likely, nestled in crevices and empty shells in a horizontal position (Beu & Maxwell Citation1990).
Remarks
Clavagella maxwelli is only the second fossil species of the genus recorded from New Zealand and the third from the southern hemisphere, the other being C. majorina BJ Smith, Citation1971 from the Oligocene of southern Australia. As noted above, BJ Smith (Citation1971) described other species of Clavagella, but Morton (Citation2007) relocated all these in other genera. Superficially, the tube of Clavagella maxwelli is similar to that of Stirpulina ramosa (Morton Citation2006b) but the anterior tubules of the adventitious tube of C. maxwelli are not arranged in a distinctive ? shape when viewed anteriorly, as they are Stirpuliniola ramosa (Morton Citation2007: fig. 17). Rather, the tubules arise haphazardly from the swollen base of the tube. Further, the tubules of other fossil Stirpulina species, e.g. S. pallinupensis from south-western Australia and Waitaki Valley (Morton Citation2006c; see below) are arranged in a distinctive anterior circlet that looks, albeit convergently, similar to the watering pot of species of the penicillid genus Penicillus (Morton Citation2006a; Citation2007: fig. 10b). Also, between the swollen region of the tube that contains the main body including the visceral mass and associated structures of species of Stirpulina and the circlet of watering pot tubules, the tube is distinctively constricted; no constriction is present in species of Clavagella.
The holotype of Clavagella oamarutica (in GNS, TM5458) comes from Gee Greensand (early Altonian, late Burdigalian, late early Miocene) at the now-buried type locality of Target Gully within the town of Oamaru. Other specimens occurring at Ngahape and Mokau on the North Island and Clifden in the southwest of the South Island range in age from Altonian to Waiauan (Late Serravallian). Specimens from the Duntroonian (Early Chattian) Chatton Formation at Chatton, near Gore, southern South Island may or may not be C. oamarutica (Maxwell Citation1978: 29; Beu & Maxwell Citation1990).
Although Clavagella maxwelli is similar in age to C. oamarutica and both have been collected from the Awamoa Beach site (Maxwell Citation1978: 29), the two species differ in the following aspects. Firstly, although the holotypes of Clavagella maxwelli and C. oamarutica have a similar tube length (c. 80mm), C. oamarutica has a maximum width of but 20 mm whereas the former is 35mm wide, i.e. it is comparatively strongly inflated anteriorly. Secondly, two fingers of secreted tube material (present in both the holotype and paratype 1 of C. maxwelli) embrace the umbo and extend over from the left side of the tube to the right. This is not the case in C. oamarutica. From between the two fingers in C. maxwelli a suture arises that unites with others on the tube's right side. Thirdly, C. maxwelli is clearly a vertically (or nearly so) burrowing endobenthic species whereas C. oamarutica was a cryptic, nestling, possibly cemented species.
BJ Smith (Citation1971: 139–140, pl. 10, figs. 6–7) described and illustrated the adventitious tube of Clavagella majorina from southern Australia. Although this species has a tube that at a length of 75mm (holotype) is similar to that of C. maxwelli (holotype=80mm), the width is much narrower, i.e. 14mm versus 35mm, respectively. The paratypes of both species are of approximately similar relative dimensions. However, C. majorina is not swollen anteriorly as in C. maxwelli; instead, the tube tapers gently from the anterior to the posterior in lateral view but is almost straight-sided in dorsal view. Maxwell (Citation1978: 21) similarly commented on the straight-sided similarity between the New Zealand species C. oamarutica and the Australian species C. majorina and considered them to be consubgeneric. It is herein considered that both species were cryptic species, either nestling or cemented, and hence unlike the vertically oriented, endobenthic species C. maxwelli.
Stoliczka (Citation1870–1871) proposed Stirpulina as a subgenus of Clavagella Lamarck, Citation1818. As a consequence, many of the fossil species of Clavagella described from Europe in particular – for example by Deshayes (1824), Sacco (Citation1901), Luković (Citation1922) and Savazzi (Citation1982b) – are actually species of Stirpulina. Forbes (1846: pl. 17, fig. 1) accurately described the new species Clavagella semisulcata from southern India but this species, although swollen anteriorly as in C. maxwelli, differs in possessing a very narrow tube posteriorly. Clavagella maxwelli is thus different from all species of clavagellid hitherto ascribed to Stirpulina and from congeners correctly assigned to Clavagella.
Adventitious tube formation
As described above, the holotype and paratype 1 of Clavagella maxwelli are characterised by the umbo of the visible left valve, incorporated into the structure of the adventitious tube, being overgrown by two fingers of tube material, between which a suture runs down the right side of the tube to approximately the midpoint of its dorsoventral height. This joins an axial suture extending forwards from a point directly opposite the posterior end of the left valve and weaves forwards among the crown of anterior tubules to the centre of the base of the watering pot (). The course of the suture is illustrated (A) as thick black lines. B is an interpretation of the way in which the sutures represent the eventual unification of the components of the adventitious tube secreted by four regions (1–4) of the underlying mantle.
Genus Stirpulina Stoliczka, Citation1870
Stirpulina Stoliczka Citation1870: 27. Type species (by original designation): Clavagella coronata Deshayes, Citation1824, Eocene, ‘Lisy, près Meaux, et PauliaeBordeaux’, SW France (Morton Citation2006c: 104).
Diagnosis
Stirpulina differs from Clavagella and its subgenera in being endobenthic, rather than endolithic (Morton Citation1984a; Citation2006c). Stirpulina is therefore the only clavagellid that builds a free tube rather than one supported in its sediment or rock substratum. The ‘watering pot’ tubules also are arranged around the anterior-most end of the swollen visceral region only in a curve in Stirpulina and are separated from the swollen tube by a marked constriction, rather than being distributed evenly but haphazardly around the similarly evenly swollen visceral region of the tube as in Clavagella.
Stirpulina cf. pallinupensis Morton, 2006 A–C.
Stirpulina pallinupense (sic) Morton Citation2006c:104, figs.1–3.
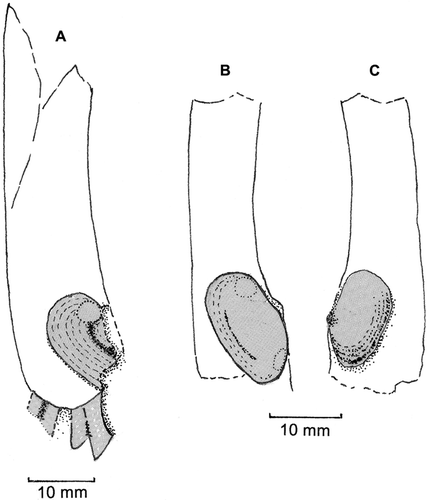
Figure 5 Stirpulina cf. pallinupensis Morton, OU40752, Wharekuri Greensand (Whaingaroan, Oligocene), Wharekuri Creek, Waitaki Valley, North Otago; drawings of latex casts of internal moulds. A, Specimen 1, showing basal portions of four unbranched, rather flat tubules, and posterior two-thirds of interior surface of free right valve. (B, C) Specimen 2, showing interior surfaces of B, right valve and C, left valve.
Type material
Holotype WAM 94.524, Western Australian Museum, Perth; from Pallinup Formation (Late Eocene), Esperance, Western Australia (Morton Citation2006c: figs. 1A, B, 2, 3). One other specimen (WAM 10430) from Pallinup Formation is possibly conspecific, but lacks the true shell and is not identifiable (Morton Citation2006c: 106).
Material examined
OU40752, Geology Department, Otago University, two latex internal casts of partial clavagellid tubes from 10 m downstream of highway culvert, Wharekuri Creek, Waitaki Valley, North Otago, New Zealand, collected by RE Fordyce, 28 May1992, from Wharekuri Greensand, Whaingaroan NZ stage (Rupelian–early Chattian, Oligocene).
Description
Specimen 1 (A) comprises a tube c. 65mm long (including the longest watering pot tubule) with, anteriorly, the basal portions of four unbranched, rather flat tubules. Only the posterior two-thirds of the interior surface of the free right valve is preserved. It is, however, oriented in a postero-ventral direction, possesses distinct commarginal growth lines, and the outline of the posterior adductor muscle scar seems to be identifiable. In life, it is estimated the valve would have been c. 12mm long and c. 10mm in height. There is no indication of a left valve.
Specimen 2 (B, C) comprises a tube c. 45mm long with no anterior tubules. The interior surface of the right valve (B) is c. 17mm long and c. 10mm in height. The interior surface of the left valve (which in life would be incorporated into the fabric of the adventitious tube; C) is c. 14mm long and c. 8mm in height. Both valves are oriented postero-ventrally and commarginal growth lines are obvious.
Remarks
There is a disparity in the sizes of (i) the two shells and (ii) between those of the right and left valves of specimen 2. Such discrepancies are explained by (i) the adventitious tubes can be formed over a narrow range of shell lengths when a habitat suitable for final occupation is chosen and (ii) subsequent to the tube being formed (specimen 2) the free, internal right valve can continue growing whereas the left valve cannot as it is incorporated into the structure of the tube, as in the extant Japanese species Stirpulina ramosa (Morton Citation2006b).
In all respects, save for the lengths of the adventitious tubes (both of which are broken anteriorly and posteriorly) the two specimens are clearly either conspecific with or very similar to the holotype of Stirpulina pallinupensis (Morton Citation2006a: 104, figs. 2, 3). Clavagellid tube length is highly variable because (i) being delicate it is easily broken and (ii) it may continue to grow in posterior length with age and to be repaired. Differences in tube lengths between the holotype of S. pallinupensis and the present specimens are therefore not significant. The similarities in shell size, shell orientation and anterior tubule format suggest conspecificity between the Australian and New Zealand specimens although, bearing in mind the age discrepancy between the two sets of specimens, this view is tempered.
We therefore have (i) the first record of the genus Stirpulina from New Zealand and (ii) a record of a species either conspecific with or closely allied to Stirpulina pallinupensis, hitherto recorded only from Western Australia.
Distribution
Stirpulina pallinupensis (possibly including a distinct, very similar species) is recorded from the Pallinup Formation (Late Eocene) in south-western Western Australia and from the Wharekuri Greensand (Whaingaroan, early Oligocene) in the Waitaki Valley, New Zealand.
Discussion
Savazzi (Citation1999) first showed how the sutures that characterise the adventitious tube of the fossil Stirpulina coronata result from the fusion of secretions produced from the underlying mantle and thereby create its unique structure. The tube of the extant species Stirpuliniola ramosa is formed in a similar manner to create a C-shaped watering pot when viewed from the anterior aspect (Morton Citation2006b). This study shows () that the adventitious tube of Clavagella maxwelli is formed in the same way as that of the American fossil clavagellid Ascaulocardium armatum (Pojeta & Sohl Citation1987), thereby attesting to the integrity of the Clavagellidae. In contrast, the adventitious tubes of all representatives of the Penicillidae are formed in a wholly different manner (Morton Citation1984b; Harper & Morton Citation2004) so that there are no sutures and the watering pot is bilaterally symmetrical (Morton Citation2007). Similarly, the crypts of epibenthic, nestling species of both families, i.e. Dianadema minima GB Sowerby III, 1889 (Clavagellidae) and Humphreyia strangei (A Adams, 1852) (Penicillidae) are secreted in analogous manners (Morton Citation2002b; Citation2009). The two families thus represent a remarkable example of convergent evolution (Morton Citation2007), both probably being independently evolved from lyonsiid ancestors at different times: the Penicillidae during the Oligocene and the Clavagellidae during the Late Cretaceous. Similarly, Stirpulina (arguably the most advanced clavagellid) is convergently similar to Penicillus, arguably the most advanced penicillid (Morton Citation2006a). But it is also true that the haphazardly arranged array of watering pot tubules that characterises the most primitive clavagelloids, i.e. species of Clavagella, find concordance with the most primitive penicillid Nipponoclava (Morton Citation2004a; Citation2007).
The New Zealand clavagellids Clavagella oamarutica (Maxwell Citation1978; Beu & Maxwell Citation1990) and C. maxwelli (described here) date from the late Early Miocene. The Australian species C. majorina dates from the Late Oligocene (BJ Smith Citation1971). Other Australian clavagellids include Dianadema liratum (Tate, Citation1887) (Darragh Citation1970) from the Upper Eocene Blanche Point Marl of South Australia (Lamprell & Healy Citation1998), although BJ Smith (Citation1971) and Cotton (Citation1952) recorded this species (the latter as Humphreyia liratum) from: the Middle Miocene Muddy Creek Formation, near Hamilton, western Victoria; the Oligocene at Torquay, southern Victoria; and the Lower Pliocene of the Adelaide Plains area of South Australia (some of these records possibly represent distinct species). Morton (Citation2006c) recorded the endobenthic clavagellid Stirpulina pallinupensis from the late Eocene Pallinup Formation of the Bremer Basin, southern Western Australia, and it is also recorded here from Oligocene rocks in New Zealand.
Among the Late Eocene fossil assemblages from the Pallinup Formation at Walpole, south-western Western Australia, most of the bivalve genera have a cosmopolitan distribution with either related or similar species occurring in New Zealand, Asia, Europe and America (Darragh & Kendrick Citation1980; Citation2000). This fossil record suggests that during the Eocene some molluscan elements of the Tethyan–Southwest Pacific fauna and other more cosmopolitan groups extended their ranges into the southern hemisphere, including Australian and New Zealand waters, arriving in the latter during the Miocene. Clavagella maxwelli conforms to this dating, thereby again suggesting a clavagellid invasion of the southern hemisphere somewhat prior to this time. This invasion seems to have resulted in the establishment of two clavagellid clades in the southern hemisphere, represented by the vertically oriented endobenthic species Clavagella maxwelli and Stirpulina pallinupensis in New Zealand and Australia and the cryptic, variously oriented, nestling, possibly cemented, Clavagella oamarutica and C. majorina in New Zealand and Australia, respectively. It should be pointed out, however, that the Palaeocene–Eocene fossil record of New Zealand and Australia is neither complete nor diverse enough to record whether or not clavagellids were present during that time.
During the Late Eocene some molluscan elements of the Tethyan–Southwest Pacific fauna, probably including Stirpulina pallinupensis and other more cosmopolitan groups, extended their ranges into southern Australian waters. They joined southern endemic species to form a distinctive blended fauna (Darragh & Kendrick Citation1980; Citation2000) such as is found today in the Pallinup Formation of south-western Western Australia. The Pallinup Formation of the Bremer Basin (Gammon et al. Citation2000) was deposited on the shallow, inner continental shelf (Darragh & Kendrick Citation1980) and the co-occurrence here of S. pallinupensis and in the Wharekuri Greensands of southern New Zealand suggests a similar shallow-water habitat. With the Oligocene, there was a general decline in South Pacific sea temperatures (Shackleton & Kennett Citation1975) and opening of the Drake Passage facilitating the circum-Antarctic circulation in the Southern Ocean (Beu et al. Citation1997). As a consequence, the warmer-water molluscan assemblages of the Late Eocene in southern Australia were succeeded by a cooler-water Oligocene community (Darragh Citation1985). Thus, the Pectinidae show a substantial faunal disjunction in southern Australia from the Late Eocene to the Oligocene (Janjukian), consistent with a cooling sea. The marine fauna of New Zealand was probably affected in a similar manner (Beu & Darragh Citation2001). This also appears to be the case with Stirpulina pallinupensis, the fossil specimens reported here upon being recorded from the Oligocene (c. 28 Ma) but not more recently. There is, however, an age discrepancy of c. 10 million years between the Western Australian (Late Eocene, Priabonian) and New Zealand (Oligocene, early Chattian) records of S. pallinupensis. In view of the rarity of fossil clavagellids and the scarcity of fossil shallow-marine faunas in the Palaeocene and Eocene of New Zealand and southern Australia, evidence is lacking for the total time and geographical ranges of S. pallinupensis.
Acknowledgements
This article is dedicated to the memory of Phillip Maxwell and Andrew Grebneff. Phil told AG that ‘I think I saw a couple of clavagellids’ after one visit to the Awamoa Beach locality. These were still in place when AG went to collect them, and became the holotype and paratype 1. Prof. R Ewan Fordyce, Department of Geology, University of Otago, kindly dated foraminiferans from a matrix sample from Awamoa Beach, and provided the specimens of Stirpulina to BM. BM is also grateful to George Kendrick, Curator Emeritus, Western Australian Museum, Perth, for his helpful comments on the first draft of the manuscript of this paper.
References
- Appukuttan , KK . 1974 . Rediscovery of Clavagella (Bryopa) lata (Clavagellidae, Bivalvia) from the Gulf of Mannar, southeast coast of India . Journal of the Malacological Society of Australia , 3 : 19 – 24 .
- Beu , AG and Maxwell , PA . 1990 . Cenozoic Mollusca of New Zealand . New Zealand Geological Survey Paleontological Bulletin , 58 : 1 – 518 .
- Beu , AG and Darragh , TA . 2001 . Revision of southern Australian Cenozoic fossil Pectinidae (Mollusca: Bivalvia) . Proceedings of the Royal Society of Victoria , 113 : 1 – 205 .
- Beu , AG , Griffin , M and Maxwell , PA . 1997 . Opening of Drake Passage and late Miocene to Pleistocene cooling reflected in Southern Ocean molluscan dispersal: evidence from New Zealand and Argentina . Tectonophysics , 281 : 83 – 97 .
- Children JG 1822–1823 . Lamarck's genera of shells . The Quarterly Journal of Science, Literature, and the Arts 14(27): 64–87, pls 3, 4, Oct. 1822; 14(28): 298–322, pls 5, 6, Jan. 1823; 15(29): 23–52, pls 2, 3, Apr. 1823; 15(30): 216–258, pls 7, 8, July 1823; 16(31): 49–79, pl. 5, Oct. 1823; 16(32): 241–264, pl. 6, ‘Jan.1824’ [Dec. 1823] .
- Cotton , BC . 1952 . Appendix IV. The Mollusca of the Adelaidean Stage. In: Miles KR ed. Geology and Underground Water Resources of the Adelaide Plains Area . Geological Survey of South Australia Bulletin , 27 : 239 – 243 .
- Darragh , TA . 1970 . Catalogue of Australian Tertiary Mollusca (except chitons) . Memoirs of the National Museum of Victoria , 31 : 125 – 212 .
- Darragh , TA . 1985 . Molluscan biogeography and biostratigraphy of the Tertiary of southeastern Australia . Alcheringa , 9 : 83 – 116 .
- Darragh , TA and Kendrick , GW . 1980 . Eocene bivalves from the Pallinup Siltstone near Walpole, Western Australia . Journal of the Royal Society of Western Australia , 63 : 5 – 20 .
- Darragh , TA and Kendrick , GW . 2000 . Eocene bivalves and gastropods from the Pallinup Siltstone, Western Australia, with new records from the Eocene and Oligocene of southeastern Australia . Proceedings of the Royal Society of Victoria , 112 : 17 – 58 .
- Deshayes GP 1824 . Description des Coquilles Fossiles des environs de Paris . Tome Premiere, Conchifères . Paris . 814
- Forbes E 1846 . Report on the fossil Invertebrata from southern India, collected by Mr. Kaye and Mr. Cunliffe . Transactions of the Geological Society of London Series 2 , 7 : 97 174 , map, pls 7–9 .
- Gammon , PR , James , NP , Clarke , JDA and Bone , Y . 2000 . Sedimentology and lithostratigraphy of Upper Eocene sponge-rich sediments, southwestern Western Australia . Australian Journal of Earth Sciences , 47 : 1087 – 1103 .
- Habe , T . 1952 . Pholadomyidae, Clavagellidae, Pandoridae, Juliidae, and Condylocardiidae in Japan. In: Kuroda T ed . Illustrated Catalogue of Japanese Shells , 1 ( 18 ) : 121 – 129 .
- Habe , T . 1977 . Systematics of Mollusca in Japan. Bivalvia and Scaphopoda , xiii+372 Tokyo : Hokuryukan Publishing Co .
- Harper , EM and Morton , B . 2004 . Tube construction by the watering pot shell Brechites vaginiferus (Bivalvia; Anomalodesmata; Clavagelloidea) . Acta Zoologica , 85 : 149 – 161 .
- Jones , DS and Nicol , D . 1989 . Eocene clavagelloids (Mollusca: Pelecypoda) from Florida: the first documented occurrence in the Cenozoic of the western hemisphere . Journal of Paleontology , 63 : 320 – 323 .
- Keen , AM and Smith , LA . 1969 . “ Superfamily Clavagellacea d'Orbigny, 1844 ” . In Treatise on invertebrate paleontology, Part N. Mollusca 6 , Edited by: Moore , RC . N857 – N859 . Bivalvia : Lawrence, Kansas, Geological Society of America & University of Kansas Press .
- Kilburn , RN . 1974 . A new species of Clavagella s. s. (Bivalvia: Clavagellidae) from Natal, South Africa . Journal de Conchyliologie , 111 : 89 – 92 .
- Kossmatt F 1897 . The Cretaceous deposits of Pondicherri (translated by AH Foord and Mrs AH Foord) . Records of the Geological Survey of India 30 : 51 110 , pls 6–10 .
- Kuroda T , Habe T , Oyama K 1971 . The sea shells of Sagami Bay, collected by His Majesty the Emperor of Japan . Tokyo , Maruzen Publishing Co . xvi+741 (Japanese)+489 (English)+41 p .
- Lamarck JBPA deMde 1818 . Histoire naturelle des animaux sans vertèbres … Vol. 5 . Paris , Verdière, Deterville et chez author . 612
- Lamprell K , Healy J 1998 . Bivalves of Australia. Vol. 2 . Leiden , Backhuys Publishers . 288
- Lucović , MT . 1922 . The Eocene molluscan fauna from the area between the Aral Sea and Lake Chalkar and its importance . Annales Géologiques de la Péninsule Balkanique , 8 : 22 – 82 .
- Maxwell , PA . 1978 . Taxonomic and nomenclatural notes on some New Zealand Cenozoic Mollusca, with descriptions of new taxa . New Zealand Journal of Zoology , 5 : 15 – 46 .
- Morton , B . 1984a . The biology and functional morphology of Clavagella australis (Bivalvia: Anomalodesmata) . Journal of Zoology, London , 202 : 489 – 511 .
- Morton , B . 1984b . Adventitious tube construction in Brechites vaginiferus (Bivalvia: Anomalodesmata: Clavagellacea) with an investigation of the juvenile of ‘Humphreyia strangei’ . Journal of Zoology, London , 203 : 461 – 484 .
- Morton , B . 2002a . Biology and functional morphology of the watering pot shell Brechites vaginiferus (Bivalvia: Anomalodesmata: Clavagelloidea) . Journal of Zoology, London , 257 : 545 – 562 .
- Morton , B . 2002b . The biology and functional morphology of Humphreyia strangei (Bivalvia: Anomalodesmata: Clavagelloidea): an Australian cemented ‘watering pot’ shell . Journal of Zoology, London , 258 : 11 – 25 .
- Morton , B . 2003 . The biology and functional morphology of Dianadema gen. nov. multangularis (Tate, 1887) (Bivalvia: Anomalodesmata: Clavagellidae) . Journal of Zoology, London , 259 : 389 – 401 .
- Morton , B . 2004a . The biology and functional morphology of Nipponoclava gigantea: clues to the evolution of tube dwelling in the Penicillidae (Bivalvia: Anomalodesmata: Clavagelloidea) . Journal of Zoology, London , 264 : 1 – 15 .
- Morton , B . 2004b . The biology and functional morphology of Kendrickiana gen. nov. veitchi (Bivalvia: Anomalodesmata: Clavagelloidea) from southern Australia . Invertebrate Biology , 123 : 244 – 259 .
- Morton , B . 2004c . The biology and functional morphology of Foegia novaezelandiae (Bivalvia: Anomalodesmata: Clavagelloidea) from Western Australia . Malacologia , 46 : 37 – 55 .
- Morton , B . 2005 . Biology and functional morphology of a new species of endolithic Bryopa (Bivalvia: Anomalodesmata: Clavagelloidea) from Japan and a comparison with fossil species of Stirpulina and other representatives of the Clavagellidae . Invertebrate Biology , 124 : 202 – 219 .
- Morton , B . 2006a . The functional morphology of Penicillus philippinensis (Anomalodesmata: Clavagelloidea) and the evolution of a unique muscular system in the Bivalvia . Records of the Western Australian Museum , 23 : 175 – 192 .
- Morton , B . 2006b . The structure and formation of the adventitious tube of the Japanese watering pot shell Stirpulina ramosa (Bivalvia: Anomalodesmata: Clavagellidae) and a comparison with that of the Penicillidae . Invertebrate Biology , 125 : 233 – 249 .
- Morton , B . 2006c . A new species and first record of the endobenthic clavagellid Stirpulina (Bivalvia: Anomalodesmata) from the Late Eocene of southern Western Australia . Alcheringa , 30 : 101 – 108 .
- Morton , B . 2007 . The evolution of the watering pot shells (Bivalvia: Anomalodesmata: Clavagellidae and Penicillidae) . Records of the Western Australian Museum , 24 : 19 – 64 .
- Morton , B . 2009 . A re-description of Dianadema minima (Bivalvia: Anomalodesmata: Clavagellidae) with an interpretation of adventitious crypt formation . Invertebrate Biology , 128 : 252 – 260 .
- Nicol , D . 1968 . A new Meiocardia (Pelecypoda, Glossidae) from the Eocene of Florida . The Nautilus , 81 : 89 – 93 .
- Owen , R . 1835 . On the anatomy of Clavagella Lam . Transactions of the Zoological Society of London , 1 : 269 – 274 .
- Pojeta , J Jr and Sohl , NF . 1987 . Ascaulocardium armatum (Morton, 1833), new genus (Late Cretaceous): the ultimate variation on the bivalve paradigm . The Paleontological Society Memoir , 24 : 1 – 77 .
- Pojeta , J Jr and Sohl , NF . 1988 . Eocene clavagellids from Florida . Journal of Paleontology , 62 : 8 – 26 .
- Sacco F 1901 . I mollusci dei terreni terziarii del Piemonte e della Liguria. Parte 29 . (Donacidae, Psammobiidae, Solenidae, Mesodesmidae, Mactridae, Cardiidae, Myidae, Corbulidae, Glycymeridae, Gastrochaenidae, Pholadidae, Teredidae, Cryptodontidae, Ungilinidae, Lucinidae, Tellinidae, Scrobiculariidae, Cuspidariidae, Solenomyidae, Pandoridae, Verticordiidae, Lyonsiidae, Ceromyidae, Arcomyidae, Anatainidae, Poromyidae, Clavagellidae) . Torino , C Clausen . 216
- Savazzi , E . 1982a . Adaptations to tube dwelling in the Bivalvia . Lethaia , 15 : 275 – 297 .
- Savazzi , E . 1982b . Clavagellacea (Bivalvia) from the Tertiary of the Venetian region, NE Italy . Acta Geologica Polonica , 32 : 83 – 91 .
- Savazzi , E . 1999 . “ Boring,nestling and tube-dwelling bivalves ” . In Functional morphology of the invertebrate skeleton , Edited by: Savazzi , E . 205 – 237 . Chichester, , UK : John Wiley .
- Savazzi , E . 2000 . Morphodynamics of Bryopa and the evolution of clavagelloids. In: Harper EM, Taylor JD, Crame J eds. The evolutionary biology of the Bivalvia . Geological Society of London Special Publication , 177 : 313 – 327 .
- Shackleton , NJ and Kennett , JP . 1975 . Paleotemperature history of the Cenozoic and the initiation of Antarctic glaciation: oxygen and carbon isotope analyses in DSDP sites 277, 279, 281 . Initial Reports of the Deep Sea Drilling Project , 29 : 743 – 755 .
- Smith , BJ . 1971 . A revision of the family Clavagellidae (Pelecypoda, Mollusca) from Australia with descriptions of two new species . Journal of the Malacological Society of Australia , 2 : 135 – 161 .
- Smith , BJ . 1976 . Revision of the Recent species of the family Clavagellidae (Mollusca: Bivalvia) . Journal of the Malacological Society of Australia , 3 : 187 – 209 .
- Smith BJ 1998 . Superfamily Clavagelloidea . In: Beesley PL , Ross GJB , Wells A Mollusca: the southern synthesis. Fauna of Australia 5, Part A , pp. 412 – 415 . Melbourne , CSIRO .
- Smith , LA . 1962a . Revision of the Clavagellacea . The Veliger , 4 : 167 – 174 .
- Smith , LA . 1962b . Historical zoogeographic study of the Clavagellacea . The Veliger , 5 : 15 – 19 .
- Soliman , GN . 1971 . On a new clavagellid bivalve from the Red Sea . Proceedings of the Malacological Society of London , 39 : 389 – 397 .
- Stallwood , RB . 1995 . A Turonian clavagellid (Bivalvia) from the Ladd Formation of southern California . Journal of Paleontology , 69 : 84 – 88 .
- Stoliczka , F . 1870 . 1871. The Cretaceous fauna of southern India III. The Pelecypoda with a review of all known genera of this class, fossil and Recent . Memoirs of the Geological Survey of India, Palaeontologica Indica , 3 : 1 – 538 .
- Sundaram , R , Henderson , RA , Ayyasami , K and Stilwell , JD . 2001 . A lithostratigraphic revision and palaeoenvironmental assessment of the Cretaceous System exposed in the offshore Cauvery Basin, southern India . Cretaceous Research , 22 : 743 – 762 .
- Tate , R . 1887 . The lamellibranchs of the Older Tertiary of Australia. (Part II.) . Transactions of the Royal Society of South Australia , 9 : 142 – 189 .