ABSTRACT
Elephant seals (Phocidae: Miroungini) are conspicuous members of modern marine mammal communities but have a meagre fossil record, obscuring the origins of this charismatic clade. We describe the oldest record for the group, CD 35, representing fragments of the maxilla, squamosal, as well as the P2, P3, and possible P1 from the Late Pliocene of the Petane Formation of New Zealand. This fossil possesses several features found only in Miroungini, including simplified triple-cusped teeth with smooth enamel that lack a cingulum, a reduced M1 and possession of a probable prenarial shelf. It also retains plesiomorphic features absent in Mirounga, such as double-rooted P2–4, which retain the parastyle and metacone. This fossil supports a Southern Hemisphere origin for elephant seals, consistent with recent phylogenetic analyses, and refutes previous suggestions of a North Atlantic origin for the clade.
Introduction
Elephant seals are represented by two extant species, the southern elephant seal (Mirounga leonina) with a circumpolar distribution in temperate and subantarctic waters in the Southern Hemisphere, and the northern elephant seal (Mirounga angustirostris), restricted largely to the eastern North Pacific. Mirounga spp. are the largest bodied and deepest diving of all modern pinnipeds, and possess the greatest degree of sexual dimorphism of any mammal (Hindell & Perrin Citation2009). Despite being intensely studied by biologists, their fossil record is limited to a few mostly undescribed records of Middle and Late Pleistocene age, hindering understanding of elephant seal evolution. Fossils of the tribe Miroungini include many Middle to Late Pleistocene and Holocene records of Mirounga from the eastern North Pacific (Mirounga sp., cf. M. angustirostris; Miller Citation1971; Rick et al. Citation2011; Rivin Citation2014), Chile (Valenzuela-Toro et al. Citation2015) and South Africa (Avery & Klein Citation2011).
Older records of Mirounga are more dubious. Purported teeth of Mirounga sp. were reported from lower Miocene strata of Namaqualand, South Africa (Pickford & Senut Citation1997); however, these teeth are almost certainly misidentified odontocete teeth, and predate the appearance of phocids in the Southern Hemisphere by several million years. Another supposed record of a ‘Mirounga-like species’ (Barnes & Mitchell Citation1975, p. 41) are a pair of tusks with several associated highly worn single-rooted postcanine teeth (LACM 28432) from the Pliocene San Diego Formation of California (Hendey Citation1972; Barnes & Mitchell Citation1975; Briggs & Morejohn Citation1975). One canine has been longitudinally sectioned and polished, revealing a core of globular osteodentine, diagnostic for Odobenini (Deméré Citation1994), providing clear evidence that this specimen is a walrus, not an elephant seal (Boessenecker Citation2013, p. 904). The North Atlantic Pliocene phocid Callophoca obscura has been considered to be closely related to Mirounga based on similarities in size and strong sexual dimorphism (de Muizon Citation1982; Koretsky & Ray Citation2008) and was recovered as sister to Mirounga in at least one recent cladistic analysis (Amson & de Muizon Citation2013). However, their phylogeny only recovered this relationship with poor support, and other studies have not placed Callophoca within Miroungini (Berta et al. Citation2015), instead placing Miroungini as the sister clade of Lobodontini (congruent with molecular analyses; Árnason et al. Citation2006; Higdon et al. Citation2007; Fulton & Strobeck Citation2010a, Citation2010b) and recovered Callophoca within a paraphyletic assemblage of stem-monachine taxa. Callophoca is represented in paleontological collections by a wealth of postcranial and cranial material, but no comprehensive study has been made of this taxon, and its phylogenetic position and even monophyly are uncertain. The limited fossil record of true Mirounga and misinterpretation of these other records have limited and confused the study of elephant seal evolution.
Many previous fossil records of miroungin seals are controversial or misidentified, however, one undescribed but occasionally cited specimen is the ‘Waipunga fragments’, a specimen including rostral and tooth fragments initially studied by Dr JA Berry in the early 20th century. This specimen was initially collected from a railroad cutting and given to Berry by Mr Henry Hill and subsequently deposited within National Palaeontology Collections (maintained by Geological and Nuclear Sciences, Wellington, New Zealand). Berry concluded that the specimen represented a new genus of ‘cystophorine’ seal (Fleming Citation1968), a subfamily that includes the North Atlantic hooded seal (Cystophora cristata) and Mirounga, likely on the basis of dorsoventrally shallow maxilla and likely presence of a prenarial shelf. Morphologic and molecular data find strong support for paraphyly of ‘Cystophorinae’, with Cystophora considered an early diverging phocine seal, and Mirounga the sister taxon to Antarctic seals (Lobodontini) within the Monachinae (King Citation1966; Berta & Wyss Citation1994; Bininda-Emonds et al. Citation1999; Fyler et al. Citation2005; Árnason et al. Citation2006; Higdon et al. Citation2007; Fulton & Strobeck Citation2010a, Citation2010b; Nyakatura & Bininda-Emonds Citation2012; Berta et al. Citation2015; however, for an alternative view see Koretsky & Rahmat Citation2013). Prior to his death, Dr Berry intended to write a manuscript formally naming the ‘Waipunga fragments’ as a new genus and species of cystophorine seal; regardless of the correct subfamilial assignment, CD 35 is too incomplete to designate as a holotype. Although unsuitable for naming, this specimen genuinely exhibits a mix of archaic monachine features and derived Mirounga-like features, and represents one of the few discovered specimens of Australasian fossil pinnipeds. The purpose of this study is to report the morphology and probable stratigraphy of this fragmentary, but historically important, oft-cited, but undescribed specimen and discuss its implications for elephant seal evolution.
Institutional abbreviations
LACM, Natural History Museum of Los Angeles County; CD, National Palaeontology Collection, Institute of Geological and Nuclear Sciences, Wellington, New Zealand.
Methods
Fragments of CD 35 were placed into approximate life position using modelling clay and subsequently coated with sublimated ammonium chloride and photographed with a Canon Rebel XS and 80 mm zoom lens. Focus stacking using Photoshop Creative Suite 5 (Adobe) was used to create seamless photographs with continuous focus.
Locality and geological setting
The fragmentary Waipunga specimen was discovered by railway workers during construction of the Hawke’s Bay railway north of Napier (North Island, New Zealand). According to Fleming (Citation1968, pp. 1185–1186) various descriptions of the locality include ‘at Waikohou, about 20 miles north from Napier’, the ‘blue clay at Kaiwaka’ and ‘a mile or two beyond Waipunga in the direction of Mohaka’. Grant-Taylor (in Fleming Citation1968) considered the locality to be Nukumaruan, probably upper Nukumaruan. Dr JA Berry recalled that upon discovery ‘an argument ensued as to whether the find was really a fossil skull or not and it was shattered to pieces with a pickaxe, when a number of two-rooted teeth were exposed’ (Fleming Citation1968), perhaps explaining the fragmentary nature of CD 35.
No surviving town or village maintains the name Waipunga, but the Waipunga rail station (; Haywick et al. Citation1991, ) is located c. 2.5 km south of Kaiwaka; several railroad cuttings still exist near Kaiwaka. The entirety of this region is mapped as the Plio-Pleistocene Petane Formation by Lee et al. (Citation2011). The Petane Formation is locally 550 m thick and was formerly elevated to group status by Haywick et al. (Citation1991). Only two units within the Petane Formation consist of blue mudrocks: the ‘Esk Formation’ and stratigraphically higher ‘Marau Formation’ (Haywick et al. Citation1991). Kaiwaka is located at the extreme western edge of detailed mapping by Haywick et al. (Citation1991, Figure 5), and the railway locally appears to be at or below the level of the conglomeratic ‘Tutira Formation’, which directly overlies the ‘Esk Formation’. Because the ‘Esk Formation’ is exposed locally and is the only unit near the railway including blue mudrocks, CD 35 was probably collected from this unit. The ‘Esk Formation’ consists of uncemented, massive to intensely bioturbated blue–grey sandy silt deposited on the mid-outer continental shelf (50–200 m depth) and preserves a marine invertebrate fauna indicating open marine conditions (Haywick et al. Citation1991). The upper ‘Esk Formation’ includes the last occurrence of Globorotalia crassiformis (dextral) (Haywick et al. Citation1991), the extinction of which is dated to 2.1 Ma (Carter et al. Citation1999, table T9). The base of the Petane Formation is no older than Nukumaruan (2.4–1.64 Ma) and thus the age of the Waipunga seal can be summarized as Late Pliocene (2.4–2.1 Ma) following the traditional definition of the Pliocene–Pleistocene boundary as recommended by Hilgen et al. (Citation2012). For geologic maps of the region and a stratigraphic column of the Petane Formation (sensu Lee et al. Citation2011), see Haywick et al. (Citation1991, p. 217) and Lee et al. (Citation2011).
Systematic palaeontology
Class Mammalia Linnaeus, 1758
Order Carnivora Bowdich, 1821
Suborder Pinnipedia Illiger, 1811
Family Phocidae Gray, 1821
Subfamily MonachinaeTrouessart, 1897
Tribe Miroungini Muizon, 1981
Miroungini gen. et sp. indeterminate
Referred specimen
CD 35, a fragmentary cranium including fragments of the right maxilla and squamosal, loose teeth including right P2, P3 and possible P1.
Locality and horizon
Railroad cutting near Waipunga rail station, Hawke’s Bay, New Zealand; Esk Formation within Petane Group of Haywick et al. (Citation1991; =Petane Formation of Lee et al. Citation2011), Late Pliocene (2.4–2.1 Ma). Fossil record number V20/f0638 (New Zealand Fossil Record File, Geological Society of New Zealand).
Description
Maxilla
Three fragments of the maxilla are preserved, including much of the dorsal margin, part of the alveolar margin and a fragment with the M1 alveolus and base of the zygomatic process (A–D). The three fragments do not preserve any contacts, but are reassembled based upon the probable diameter of the canine (12–15 mm; determined by fitting a cylinder of clay into the alveolus as preserved in the dorsal fragment) and the probable anteroposterior diameter of the P4 (assumed to be similar to the 8 mm long P3 alveolus). When assembled under these assumptions, the maxilla is triangular, elongate and dorsoventrally shallow in lateral view with a straight dorsal margin (A, D). The lateral surface is flat except anteriorly around the distal canine alveolus. The entire dorsal margin of the maxilla is straight (in lateral view) at least to the level of the P4; there is no dorsomedial curvature of the maxilla or dorsal termination of the medial sutural surface for the premaxilla, indicating that the narial fossa extended posteriorly at least to the level of P4. The dorsal part of the maxilla is a transversely narrow crest that is increasingly narrow and sharp posteriorly (C); medially the maxilla bears a smooth, flat articular surface for the premaxilla.
Figure 2. Preserved elements of the Waipunga Seal, Miroungini gen. et sp. indet., CD 35. A, Fragments of right maxilla with P3 and possible P2 root in approximate life position in lateral view with speculative margins of rostrum (dashed lines). B, Fragments of right maxilla in ventral view. C, Right maxilla fragments in dorsal view. D, Rostral fragments of maxilla in medial view. E, Left squamosal in ventral view. F, Left squamosal in dorsal view. G, Right P3 in labial view. H, Right P2 in lingual view. I, Right P2 in labial view. J, Right P2 in apical view.
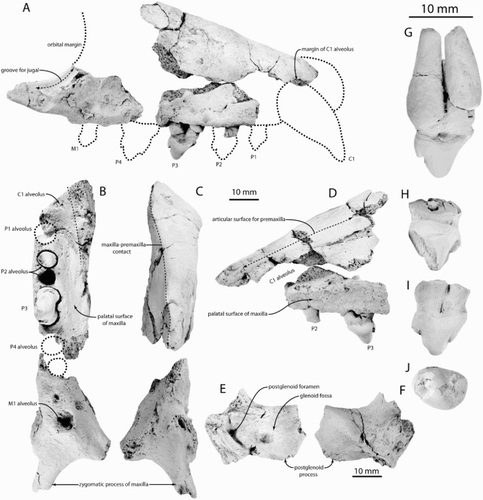
The zygomatic process of the maxilla is delicate and underlies an apparently large and ventrally situated orbit (below the dorsal margin of the canine alveolus). A dorsal groove parallel to the orbital margin and a small pit ventral to this indicate a double-headed jugal and a horizontal V‐shaped maxillojugal articulation, unlike the splint-like anterior jugal of Lobodon, Hydrurga, Leptonychotes and possibly Ommatophoca. The infraorbital foramen is small and situated anteriorly to the articular groove for the jugal. The lateral part of the palate is preserved medial to the alveoli and indicates a deeply vaulted palate (at least 12 mm deep adjacent to P4; D). Posteriorly (near M1) the palate is concave but shallower than anteriorly. The toothrow is relatively straight (B).
Squamosal
The squamosal fragment (E–F) preserves a deeply concave glenoid fossa and an arcuate, vertical postglenoid process. A small postglenoid foramen is present medially; the broken remnant of the tympanic bulla indicates that it was inflated and anterolaterally appressed against the postglenoid process, separated by a narrow fissure. The squamosal bears a distinct supramastoid crest above the external auditory meatus, unlike Acrophoca, Lobodon and Piscophoca.
Dentition
The C1 alveolus is incomplete but anteriorly directed and relatively large (c. 12–15 mm diameter); the alveolus extends posteriorly in the maxilla to the level of the P3. The dorsal margin of the canine alveolus aperture is preserved and c. 17 mm above the postcanine alveolar margin, suggesting that the emergent portion of the canine was relatively large. Alveoli indicate that five postcanine teeth (P1–4 and M1) were present as in all Phocidae (B). All postcanine alveoli are slanted slightly posteriorly, and when placed into its alveolus, the P3 is posteriorly inclined (A, D). The posterior margin of the P1 alveolus indicates a cylindrical root; a 3 mm diastema is present between P1 and P2. A partial cylindrical root missing a crown with an inflated root may represent P1 or the anterior root of P2 as it fits well within that alveolus (A–B, D).
The P2 alveolus indicates that it was double-rooted with a slightly wider anterior root; both roots were circular in cross section. The crown and apical root of P2 is preserved (H–J); the roots are apically fused with a deep labial sulcus. The turnip-shaped crown is oval in apical view (6.3 mm transverse width) and triangular in labial view (6.6 mm crown height; 8.7 mm anteroposterior length) with a prominent paracone and a small metacone. A large parastyle was present but is mostly worn away, and a large anterior wear facet is present; a small apical wear facet is present on the metacone. A sharp posterior crista is present, but there is no labial or lingual cingulum. The crown is more convex lingually.
The P3 alveolus is double-rooted and not separated from the P2 by a diastema. The P3 (A–B, D, G), is nearly complete except for the anterior root, which was reconstructed by Dr JA Berry as a cast of the alveolus (G); the posterior root is inflated apically and tapers basally, and is anteroposteriorly broader than the crown. The crown (6.4 mm height as preserved, 6.7 mm transverse width) is oval and more anteroposteriorly elongate (9.3 mm) than P2; the crown is similarly triangular and turnip-shaped with a prominent paracone and small metacone. A large figure-of-8 shaped anterior wear facet has destroyed the anterior surface of the crown and the presence of a parastyle is uncertain. The crown is also more convex lingually.
The anterior and posterior margins of the P4 alveolus are preserved but the size is unclear, although unlikely to have differed greatly in dimension from P3. It is separated from P3 by a 2 mm diastema.
The M1 alveolus is circular and indicates a shallow single root (B), shallower than P1–3; posteriorly it bears an elevated rim. The P4–M1 diastema is long (c. 10 mm) and a minute irregular pit is present within; it is unclear whether it represents a reduced alveolus for an anterior root of M1 or a remodelled pit for a lower postcanine crown, as in Acrophoca longirostris.
Discussion and conclusions
Our redescription of the Waipunga fragments supports placement of this taxon within the Miroungini, on the basis of the following features not shared with any other monachine taxa: simplified triple-cusped upper postcanine teeth with smooth enamel and lacking cingulum, reduced M1, and possession of a probable prenarial shelf. Referral to Otariidae is not possible owing to the following features: rostral portion of maxilla dorsoventrally shallow and elongate, swollen postcanine roots, lack of lingual cingulum and termination of the toothrow anterior to the maxillary root of the zygomatic arch. The dating of this taxon to the Late Pliocene (2.4–2.1 Ma) also makes it the oldest definite record of the Miroungini. Furthermore, the Waipunga seal possesses the following features not possessed by extant Mirounga: double-rooted P2–4 (plesiomorphic), posteriorly inclined cheek teeth (autapomorphic), retention of parastyle and metacone (plesiomorphic) and probable small adult body size (plesiomorphic). This suggests that the Waipunga seal does not belong within Mirounga, but rather represents a stem-Miroungini. This suggests that the common ancestor of the genus was present in the Southern Hemisphere, radically changing the assumed biogeographic history for Miroungini.
Previous studies of phocid biogeography have assumed a close relationship between Mirounga and Callophoca, which, as discussed above, is not well supported. This has resulted in an interpretation that Miroungini evolved within the North Atlantic. This divergence would have taken place in the latest Miocene or earliest Pliocene, based on molecular clock dates for the divergence of Lobodontini and Miroungini (6.88 Ma; Fulton & Strobeck Citation2010a). Miroungines then dispersed through the Central American Seaway to the Pacific (Fyler et al. Citation2005; Árnason et al. Citation2006; Fulton & Strobeck Citation2010a). After this dispersal, the rise of the Isthmus of Panama led to changes in equatorial currents and subsequent isolation of populations that later gave rise to M. angustirostris and M. leonina, c. 2.5 Ma (Fulton & Strobeck Citation2010a). Another problematic hypothesis presented by Briggs and Morejohn (Citation1975) inferred a North Pacific origin of Mirounga based on the assumed retention of some plesiomorphic craniodental features by M. angustirostris not possessed by M. leonina; they additionally cited undescribed fossils of Mirounga including a pair of tusks from the Pliocene San Diego Formation of southern California, which are actually from a walrus (see above). Re-identification of this oft-cited specimen (LACM 28432) as a walrus indicates that elephant seals do not have a pre-Late Pleistocene fossil record in the North Pacific.
The recognition of a stem miroungine in New Zealand during the Pliocene, as well as the lack of evidence for Miroungini affinities of Callophoca (Berta et al. Citation2015), require a new interpretation of elephant seal biogeography. We posit that Miroungines and lobodontines diverged from one another in the Southern Hemisphere, and that Mirounga itself would have originated in this region. In this biogeographic scenario, Mirounga would have colonized the North Pacific by crossing the equator in the eastern Pacific, probably during a period of equatorial cooling and enhanced productivity during the Early or Middle Pleistocene, similar to the allopatric speciation event inferred in the separation of porpoises and Arctocephalus fur seals by warming equatorial waters during the Early Pleistocene (Fajardo-Mellor et al. Citation2006; Churchill et al. Citation2014). Indeed, M. angustirostris continues to breed during the austral (rather than northern) summer, attesting to recent arrival in the North Pacific (Hendey Citation1972). Because the entirety of the North Pacific fossil record of Mirounga is confined to specimens of Mirounga sp., cf. M. angustirostris from the Upper Pleistocene (130–85 ka) Palos Verdes Sand of Southern California (Miller Citation1971; Rivin Citation2014), dispersal of the ancestor of M. angustirostris to the North Pacific likely occurred no earlier than the Early or Middle Pleistocene. The Waipunga fragments are woefully incomplete, but continued sampling of Plio-Pleistocene marine rocks in New Zealand and elsewhere in the southern hemisphere has potential to discover new material of archaic Miroungini. Further discovery, description and phylogenetic analysis of fossil phocids will clarify the centre of origin and historical biogeography of the Miroungini.
Acknowledgements
We wish to thank JE Simes (Geological and Nuclear Sciences, Wellington) for loaning the Waipunga specimen during our studies in New Zealand. Thanks to RE Fordyce (University of Otago) for discussions, hosting loans, and hosting MC’s New Zealand visit. Constructive comments from Erich Fitzgerald and one anonymous reviewer helped improve this manuscript.
Associate Editor: Professor Kathy Campbell.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amson E, de Muizon C. 2013. A new durophagous phocid (Mammalia: Carnivora) from the late Neogene of Peru and considerations on monachine seal phylogeny. J Syst Palaeontol. 12:523–548. doi: 10.1080/14772019.2013.799610
- Árnason U, Gullberg A, Janke A, Kullberg M, Lehman N, Petrov EA, Väinölä R. 2006. Pinniped phylogeny and a new hypothesis for their origin and dispersal. Mol Phylogenet Evol. 41:345–354. doi: 10.1016/j.ympev.2006.05.022
- Avery G, Klein RG. 2011. Review of fossil phocid and otariid seals from the southern and western coasts of South Africa. Trans R Soc S Afr. 66:14–24. doi: 10.1080/0035919X.2011.564490
- Barnes LG, Mitchell ED. 1975. Late Cenozoic Northeast Pacific Phocidae. Conseil International pour l’Exploration de la Mer. Rapports et Procèsverbaux des Réunions. 169:34–42.
- Berta A, Kienle S, Bianucci G, Sorbi S. 2015. A re-evaluation of Pliophoca etrusca (Pinnipedia, Phocidae) from the Pliocene of Italy: phylogenetic and biogeographic implications. J Vert Paleontol. 35:e889144. doi:10.1080/02724634.2014.889144
- Berta A, Wyss AR. 1994. Pinniped phylogeny. Proc San Diego Soc Nat Hist. 29:33–56.
- Bininda-Emonds ORP, Gittleman JL, Purvis A. 1999. Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia). Biol Rev. 74:143–175. doi: 10.1017/S0006323199005307
- Boessenecker RW. 2013. A new marine vertebrate assemblage from the Late Neogene Purisima Formation in Central California, part II: pinnipeds and cetaceans. Geodiversitas. 35:815–940. doi: 10.5252/g2013n4a5
- Briggs KT, Morejohn GV. 1975. Dentition, cranial morphology, and evolution in elephant seals. Mammalia. 40:199–222.
- Carter RM, McCave IN, Richter C, Carter L, Aita Y, Buret C, Di Stefano A, Fenner J, Fothergill P, Gradstein F, et al. 1999. Site 1125: productivity under the subtropical convergence on North Chatham Slope. Proc ODP, Init Repts. 181:9:1–92.
- Churchill M, Clementz MT, Boessenecker RW. 2014. Colonization of the Southern Hemisphere by fur seals and sea lions (Carnivora: Otariidae) revealed by combined evidence phylogenetic and Bayesian biogeographical analysis. Zool J Linnean Soc. 172:200–225. doi: 10.1111/zoj.12163
- Deméré TA. 1994. The family Odobenidae: a phylogenetic analysis of living and fossil forms. Proc San Diego Soc Nat Hist. 29:99–123.
- Fajardo-Mellor L, Berta A, Brownell A Jr., Boy CC, Goodall RNP. 2006. The phylogenetic relationships and biogeography of true porpoises (Mammalia: Phocoenidae) based on morphological data. Mar Mam Sci. 22:910–932. doi: 10.1111/j.1748-7692.2006.00080.x
- Fleming CA. 1968. Notes from the New Zealand Geological Survey—5: New Zealand fossil seals. New Zeal J Geol Geophys. 11:1184–1187. doi: 10.1080/00288306.1968.10420246
- Fulton TL, Strobeck C. 2010a. Multiple fossil calibrations, nuclear loci and mitochondrial genomes provide new insight into biogeography and divergence timing for true seals (Phocidae, Pinnipedia). J Biogeogr. 37:814–829. doi: 10.1111/j.1365-2699.2010.02271.x
- Fulton TL, Strobeck C. 2010b. Multiple markers and multiple individuals refine true seal phylogeny and bring molecules and morphology back in line. Proc R Soc Lond B. 277:1065–1070. doi: 10.1098/rspb.2009.1783
- Fyler CA, Reeder TW, Berta A, Antonelis G, Aguilar A, Androukaki E. 2005. Historical biogeography and phylogeny of monachine seals (Pinnipedia: Phocidae) based on mitochondrial and nuclear DNA data. J Biogeogr. 32:1267–1279. doi: 10.1111/j.1365-2699.2005.01281.x
- Haywick DW, Lowe DA, Beu AG, Henderson RA, Carter RM. 1991. Pliocene-Pleistocene (Nukumaruan) lithostratigraphy of the Tangoio block, and origin of sedimentary cyclicity, central Hawke’s Bay, New Zealand. New Zeal J Geol Geophys. 34:213–225. doi: 10.1080/00288306.1991.9514459
- Hendey QB. 1972. The evolution and dispersal of the Monachinae (Mammalia: Pinnipedia). Ann S Afr Mus. 59:99–113.
- Higdon JW, Bininda-Emonds ORP, Beck RMD, Ferguson SH. 2007. Phylogeny and divergence of the pinnipeds (Carnivora: Mammalia) assessed using a multigene dataset. BMC Evol Biol. 7:216. doi:10.1186/1471-2148-7-216
- Hilgen FJ, Lourens LJ, Van Dam JA. 2012. The Neogene period. In: Gradstein FM, Ogg JG, Schmitz M, Ogg G, editors. The geologic time scale 2012. Amsterdam: Elsevier; p. 923–978.
- Hindell MA, Perrin WF. 2009. Elephant seals: Mirounga angustirostris and Mirounga leonina. In: Perrin WF, Würsig B, Thewissen JGM, editors. Encyclopedia of marine mammals. San Diego, California: Academic Press; p. 364–368.
- King JE. 1966. Relationships of the hooded and elephant seals (genera Cystophora and Mirounga). J Zool Soc Lond. 148:465–470.
- Koretsky IA, Rahmat SJ. 2013. First record of fossil Cystophorinae (Carnivora, Phocidae): middle Miocene seals from the northern Paratethys. Riv Ital Paleontol S. 119:325–350.
- Koretsky IA, Ray CE. 2008. Phocidae of the Pliocene of eastern USA. Virginia Museum of Natural History Special Publications 6:81–140.
- Lee JM, Townsend D, Bland K, Kamp PJJ. 2011. Geology of the Hawke’s Bay Area: scale 1:250,000. Lower Hutt: Institute of Geological and Nuclear Sciences; 86 pp.
- Miller WE. 1971. Pleistocene vertebrates of the Los Angeles Basin and vicinity (exclusive of Rancho La Brea). Bull Los Angeles County Mus Nat Hist. 10:1–124.
- de Muizon C. 1982. Phocid phylogeny and dispersal. Ann S Afr Mus. 89:175–213.
- Nyakatura K, Bininda-Emonds ORP. 2012. Updating the evolutionary history of Carnivora (Mammalia): a new species-level supertree complete with divergence time estimates. BMC Biol. 10:1–31. doi: 10.1186/1741-7007-10-12
- Pickford M, Senut B. 1997. Cainozoic mammals from coastal Namaqualand, South Africa. Paleontol Afr. 34:199–217.
- Rick TC, Delong RL, Erlandson JM, Braje TJ, Jones TL, Arnold JE, Des Lauriers MR, Hildebrandt WR, Kennett DJ, Vellanoweth RL, Wake TA. 2011. Where were the northern elephant seals? Holocene archaeology and biogeography of Mirounga angustirostris. The Holocene. 21:1159–1166. doi: 10.1177/0959683611400463
- Rivin ME. 2014. The fossil record of elephant seals (Mirounga) in southern California. Secondary Adaptation of Tetrapods to Life in Water, Washington D.C., June 2–4. Unpaginated.
- Valenzuela-Toro A, Gutstein C, Suárez M, Otero R, Pyenson ND. 2015. Elephant seal (Mirounga sp.) from the Pleistocene of the Antofagasta Region, northern Chile. J Vert Paleontol. 35:e918883. doi:10.1080/02724634.2014.918883