ABSTRACT
Weathering of primary silicates to secondary clay minerals over time affects multiple soil functions such as the accumulation of organic matter and nutrient cations. However, the extent of clay mineral (trans)formation as a function of soil development is poorly understood. In this study, the degree of weathering of sediments along a 120 kyr soil formation gradient was investigated using X‐ray diffraction, Fourier transform infrared spectroscopy and X‐ray fluorescence spectroscopy. Irrespective of site age, mica and chlorite were the dominant clay minerals. During weathering, a remarkable suite of transitional phases such as vermiculite and several interstratifications with vermiculitic, smectitic, chloritic and micaceous layers developed. The degree of weathering was correlated with soil pH and depletion of K, Ca, Na, Fe and Al, regarding both soil depth and site age. Kaolinite occurred especially at the 120 kyr site, indicating slow formation via transitional phases. The findings of this study revealed that long-term soil development caused complex clay mineral assemblages, both temporally and spatially, and linking this variability to soil functioning warrants further research.
Introduction
Clay minerals are aluminosilicates composed of Si tetrahedral and Al octahedral layers separated by interlayers of variable composition, and often occur in soils and sediments as interstratified phases of different clay minerals (Rich Citation1968; Hillier Citation2001). Interstratifications are clay phases in which two or more types of silicate layer occur vertically stacked within the same crystallite or X‐ray scattering domain (Brigatti et al. Citation2013). Hydroxy-interlayered minerals (HIM) such as hydroxyl-interlayered smectite (HIS) or hydroxy-interlayered vermiculite (HIV) are typical weathering products in acidic soils and contain varying amounts of Al[(OH)x,(H2O)y]3– x polymers in the interlayer space (Barnhisel & Bertsch Citation1989; Lanson et al. Citation2015). Aluminium released by hydrolytic weathering of minerals like feldspar and mica polymerises depending on pH (Vincente et al. Citation1977; Sverdrup et al. Citation1994) and becomes intercalated in smectite or vermiculite formed by weathering from, for example, mica or chlorite (MacEwan Citation1954; Ismail Citation1969, Citation1970). Intercalation of polymeric Al species leads to an exchange of interlayer cations such as Mg, Ca or Na, thus forming HIM with basal distances of c. 14 Å. The higher the degree of hydroxy intercalation (HI), the lower the cation exchange capacity and expandability of these layers (Janssen et al. Citation1997). The most favourable conditions for the formation of HIM are moderately acidic pH (4.6–5.8), frequent wetting and drying cycles, and low organic matter content (Rich Citation1968).
In addition to HIM, interstratified clay minerals form upon silicate weathering, for example, from mica group minerals such as biotite. Bain et al. (Citation1990) showed that in Scottish upland soil, acidity and moisture were important variables governing the formation of interstratified mica-vermiculites from mica. Wilson (Citation2004) pointed out that biotite (phlogopite/annite) frequently weathers easily to form a regularly interstratified biotite-vermiculite mixed layer mineral called hydrobiotite. In fact, biotite weathered to hydrobiotite contains regular alternation of 10 Å biotitic and 14 Å vermiculitic units, thus yielding distinct X‐ray diffraction (XRD) reflections at 12 and 24 Å. However, disordered interstratified mica-vermiculites have also been reported (Coleman et al. Citation1963). In summary, the formation and alteration of interstratified clay minerals during silicate weathering, as well as the controlling soil environmental conditions, are poorly understood.
This study investigated the time- and pedogenesis-dependent formation of interstratified clay minerals along the Franz Josef soil chronosequence in Westland, New Zealand, developed from alluvial moraines at the Franz Josef Glacier. The soils developed over 120 kyr in silica-rich parent materials (schist, greywacke) giving rise to pronounced gradients in soil mineralogy and soil formation (Mokma et al. Citation1973; Turner et al. Citation2014). The metamorphic rock inventory of this alluvium originated from sedimentary rocks of different metamorphic grades (Grindley Citation1963). Grapes and Otsuki (Citation1983) described these rocks as quartzofeldspathic schists (greywackes and argillites). Depending on the metamorphic zone, these rocks contain different minerals that together form a pool of weathering products during soil formation: quartz, plagioclase, K‐feldspar, muscovite, biotite, chlorite, hornblende, epidote, ilmenite, zircon, actinolite, stilpnomelane, garnet, sphene, diaspore, ankerite, calcite, tourmaline and apatite (Mokma et al. Citation1973; Grapes & Otsuki Citation1983; Almond et al. Citation2001). Acidic weathering conditions, high annual rainfall and frequent ground frost lead to increasing rock disintegration and clay mineral formation (Mokma et al. Citation1973; Larsen et al. Citation2014). Soil pH values changed considerably over 120 kyr of soil development, ranging from 6.4 to 3.8, and increased with soil depth, whereas topsoil pH decreased with site age (Turner et al. Citation2014). Therefore, the weathering and formation conditions for clay minerals differ markedly between upper and lower soil horizons, which requires assessment of whole soil profiles. Given such extreme differences in the environmental conditions encountered in Franz Josef soils, our main objective was to investigate the formation and composition of interstratified clay minerals in topsoil and subsoil horizons over time, and to relate their abundance to the environmental conditions. Although the (clay) mineralogy and petrography of the Franz Josef chronosequence were described 40 years ago (Mokma et al. Citation1973), no work has been done on interstratified clay minerals, partly due to the poor resolution of XRD instruments at that time. Rock and soils along the chronosequence were collected and analysed for their element contents using X‐ray fluorescence spectroscopy (XRF), and isolated clay fractions were then characterised by XRD and Fourier transform infrared (FTIR) spectroscopy.
Materials and methods
Sites and soil sampling
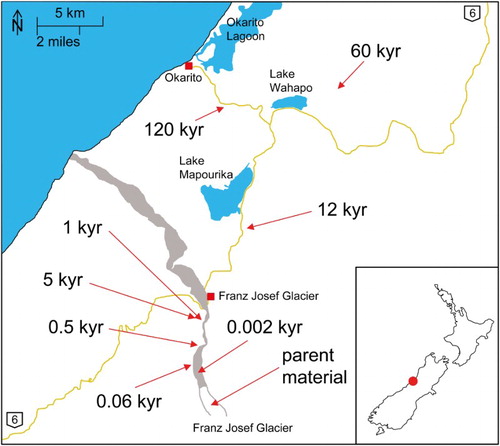
The Franz Josef Glacier is located on South Island, New Zealand (; described in detail by Turner et al. Citation2014). Within the last 120 kyr due to glacial retreat, the soils of the chronosequence formed from greywacke and mica schist and their respective weathering products (Stevens Citation1968). The two oldest sites (60 and 120 kyr) are additionally influenced by Pleistocene loess depositions (Almond et al. Citation2001). All investigated sites were covered by temperate rainforest and exposed to different amounts of precipitation, whereby the four youngest sites (0.06 to 5 kyr) receive c. 6500 mm per year, and the three oldest sites (12 to 120 kyr) c. 3500 mm per year (Richardson et al. Citation2004; Turner et al. Citation2014). According to Larsen et al. (Citation2014), the occurrence of vegetation and a high amount of precipitation promote leaching and weathering. The sites used in this study (; Table S1) were dated by Stevens (Citation1968) and Almond et al. (Citation2001) using soil stratigraphy, pollen analyses, radiocarbon dating, luminescence dating of loess and correlation with global climatic events. Although fluctuations in the Franz Josef Glacier retreat are documented, there are few sources of information about its retreat (Stevens Citation1968). Almond et al. (Citation2001) state that soil dating was not unambiguous because, for example, thermos-luminescence and optical dating yielded low-precision ages that contradict stratigraphy and radiocarbon ages. These authors related these problems to the highly weathered state of the soil samples, and dates used in this study are, therefore, approximate. However, because this study addresses the formation and transformation of clay mineral phases in relation to relative age, variations absolute ages are not important.
Figure 1. Map of the South Island of New Zealand showing the location of the Franz Josef Glacier chronosequence sampling sites, marked with their estimated ages as determined by Stevens (Citation1968) and Almond et al. (Citation2001).
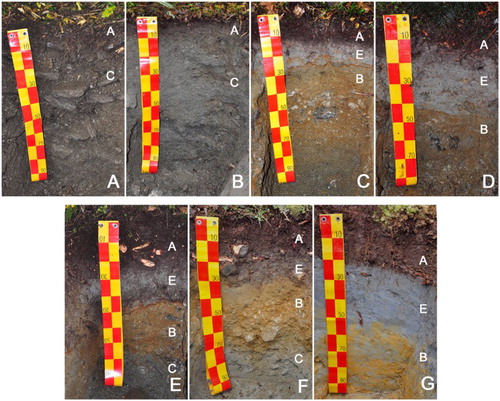
Soil samples of genetic horizons to a soil depth of 1 m were collected (; described in detail by Turner et al. Citation2014). Hereafter, for each profile, the horizons were grouped into mineral topsoil (pure and transitional A horizons), subsoil E (eluvial horizons showing metal leaching due to reductive and/or acidic conditions), subsoil B (chemically weathered horizons enriched in pedogenic Fe and Al oxides) and subsoil C (deeper horizons characterised by physical weathering). The microbial and chemical properties of the bulk materials were described by Turner et al. (Citation2014). Moist soil material was passed through a 2 mm sieve and subsequently air-dried. Bulk samples were ground in an agate mortar mill and used for mineralogical and chemical (XRF) analysis. Three procedures were used for clay mineral analysis. Thirty-three bulk soil samples were selected and treated with 6% NaOCl solution to remove organic matter without harming the mineral phase (Mikutta et al. Citation2005). These samples were then treated with dithionite-citrate-bicarbonate at 80 °C (Mehra & Jackson Citation1960) to remove pedogenic Fe oxides, followed by washing with deionised water. Clay fractions <2 µm were separated and collected fractions were saturated successively with Mg and K without further drying. After preparation on ceramic tiles, clay fractions were air-dried and used for XRD analysis. Selected samples were analysed by XRD after treatment with 1 mol/L HCl solution and heating overnight at 60 °C to investigate the types of chlorite and vermiculite present. Clay fractions of two samples, representing the source material for soil formation, were isolated and analysed without chemical pre-treatment. Gravel-sized parent material and sand-sized sediment collected from a streamlet in the outwash plane close to the glacier (exposure age 2 years, hereafter ‘river sediment’), were crushed and sonicated before clay fractions were finally separated using deionised water only. The suspensions <2 µm were oven-dried (60 °C) and re-suspended in deionised water using an ultrasonic bath.
Figure 2. Soil profiles developed along the chronosequence at: A, 0.06 kyr, B, 0.5 kyr, C, 1 kyr, D, 5 kyr, E, 12 kyr, F, 60 kyr and G, 120 kyr. Horizons are marked as A (mineral topsoil, pure and transitional A horizons), subsoil E (eluvial horizons showing metal leaching due to reductive and/or acidic conditions), subsoil B (chemically weathered horizons enriched in pedogenic Fe oxides) and subsoil C (deeper horizons characterised by physical weathering).
X‐ray diffraction of powdered and preferred oriented specimens
To investigate the bulk mineralogy of soils and parent rock materials, XRD patterns of 87 bulk samples were recorded using a PANalytical X’Pert PRO MPD Θ-Θ diffractometer (Cu‐Kα radiation generated at 40 kV and 30 mA; λα1=1.5406 Å, λα2=1.5444 Å), equipped with a variable divergence slit (20 mm irradiated length), a primary and secondary soller collimator, and a scientific X’Celerator detector. The randomly powdered samples were investigated from 2° to 85° 2Θ with a step size of c. 0.0167° 2Θ. The measuring time was 10 seconds per step. The randomly powdered samples were prepared using the back-loading technique.
XRD patterns of 33 clay fractions <2 µm were recorded to characterise clay minerals using a PANalytical X’Pert PRO MPD Θ-Θ diffractometer (Co‐Kα radiation generated at 40 kV and 40 mA; λα1=1.7890 Å, λα2=1.7929 Å), equipped with a variable divergence slit (20 mm irradiated length), a primary and secondary soller collimator, a proportional counter, and a secondary monochromator. Oriented mounts were investigated from 2° to 35° 2Θ with a step size of 0.03° 2Θ, randomly powdered samples from 1° to 80° 2Θ with a step size of 0.03° 2Θ. The measuring time was 4 and 5 seconds per step, respectively. Oriented mounts were prepared by transferring the suspended clay fractions to ceramic tiles using a vacuum filter in order to orient the clay minerals preferentially parallel to their basal planes (Dohrmann et al. Citation2009). Different conditions were applied for clay mineral analysis: (a) air-dry (AD), (b) ethylene glycol (EG) saturation, (c) AD (K) and (d) heating (K). Most of the K-saturated specimens were heated in a conventional oven for 1 hour each at 375, 450 and 550 °C. After each heat treatment, the clay films were scanned by XRD. For selected samples a heated vacuum chamber was used and K-saturated specimen were analysed at room temperature followed by scans at 240 and 375 °C, labelled hereafter K240 and K375, and again at room temperature. The randomly powdered samples were prepared using the back-loading technique.
Qualitative analysis of clay minerals was performed after Méring (Citation1949), Brindley and Brown (Citation1984), Sawhney (Citation1989), and Meunier (Citation2007). The presence or absence of reflections, their d‐values and intensity ratios were used to distinguish interstratifications and to estimate the amounts of layer types (Sawhney Citation1989; Moore & Reynolds Citation1997). Interstratifications were identified based on several (00 l) reflections, although only the most obvious reflections deemed important for interpretation are cited. The degree of HI was determined by K-intercalation (air drying and vacuum at room temperature) with subsequent heating to 375 °C. The errors of the d‐values given are estimated to ±0.002 Å for d‐values <2 Å, ±0.01 Å for d‐values between 2 and 5 Å, ±0.05 Å for d‐values between 5 and 18 Å, and ±0.3 Å for d‐values >18 Å.
Interstratifications were subdivided after different Reichweite (R)-values, if possible. The R-value of an interstratification describes the degree of long-range ordering (Jagodzinski Citation1949) as an expression of the extent of the influence of one layer on the probability of the nature of its adjacent layers (Ufer et al. Citation2012a; Brigatti et al. Citation2013). R0 means layers are stacked randomly, increasing R-values refer to an increasing long-range order, although R1 ordered or regularly stacked interstratifications were identified by investigating the rationality of the (00 l) series (Moore & Reynolds Citation1997). Discrete phases are named with mineral names (chlorite, mica, vermiculite), whereas interstratifications are named with a hyphen separating the two interstratified phases (e.g. mica-chlorite). The types of layers are termed micaceous, chloritic, vermiculitic and smectitic, respectively, to distinguish these types of layers from discrete phases. Chlorite as a mineral name refers to educt minerals, whereas chloritic layers (in interstratifications) and secondary chlorite refer to newly formed chloritic layers/chlorite.
Standard analysis techniques such as FTIR, XRF and scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) are described in Figure S1 and Table S1.
Results and discussion
Parent rock material and river sediment
The sample stated as parent material consisted of gravel-sized mica schist and greywacke (Grapes & Otsuki Citation1983). The clay fraction of this sample was assumed to represent the starting material for soil development of the profiles studied. The river sediment contained smaller grain sizes (sand) and a higher content of dark components dominated by biotite.
Non-phyllosilicates of both samples were found to be dominated by quartz and Na-rich feldspar (± albite) but traces of Fe-bearing dolomite, calcite, ilmenite and a clinopyroxene group mineral were identified by XRD (not shown) and SEM (Figure S1A–B), as typical for greywacke (Figure S1D). Traces of hornblende and K‐feldspar could not be excluded. The presence of carbonate phases in the parent material, but its absence in the river sediment indicated a very low degree of weathering of the parent material or no weathering at all, whereas the river sediment was already acidic weathered, as also supported by the presence of altered mica schist (). Larsen et al. (Citation2014) found that pervasively fractured rocks promote weathering and pedogenesis.
Figure 3. Scanning electron microscopy image of weathered mica schist rock fragments in river sediment material (fraction 2–500 µm).

The dominant phyllosilicates present in the parent rock material were found to be mica (dioctahedral and trioctahedral; Rieder et al. Citation1998; Figure S1C) and trioctahedral chlorite (Fe-rich clinochlore; XRD and FTIR detection), whereas kaolinite and halloysite were absent based on FTIR analysis. In the <2 µm fraction, chlorite and mica were dominant (), whereby dioctahedral mica with d060 = 1.506 Å and d001/002 = 9.99 Å strongly dominated over trioctahedral mica, indicating coarse-grained biotite and fine-grained dioctahedral mica. SEM–EDS analyses confirmed the presence of Fe-containing dioctahedral mica, thus fitting well with the d060 value (Moore & Reynolds Citation1997; Rieder et al. Citation1998). A broad reflection between 10 and 14 Å (AD) containing expandable layers (EG) was observed, which partially collapsed after K‐saturation and heat treatment (375 °C, vacuum) to 10–12 Å (A), indicating minor amounts of a random or short-range ordered mica-hydroxy-interlayered vermiculite (M(0.65)-HIV), and possibly traces of a mica-hydroxy-interlayered smectite (M-HIS). Traces of predominantly monovalent (one water layer) occupied vermiculite were also identified (AD 12.4 Å, rational basal series; A). Both phases are presumably formed by K‐depletion and Mg intercalation of biotite, similar to the process proposed by Banfield and Eggleton (Citation1988). The HI indicates a beginning dissolution of Al-containing phases such as feldspar.
Figure 4. XRD pattern of preferred oriented <2 µm fractions (AD, EG and K375) isolated from A, the parent rock material and B, river sediment. Chl, chlorite; Verm, vermiculite; M-HIV, mica-hydroxy-interlayered vermiculite; Chl-S, chlorite-smectite.
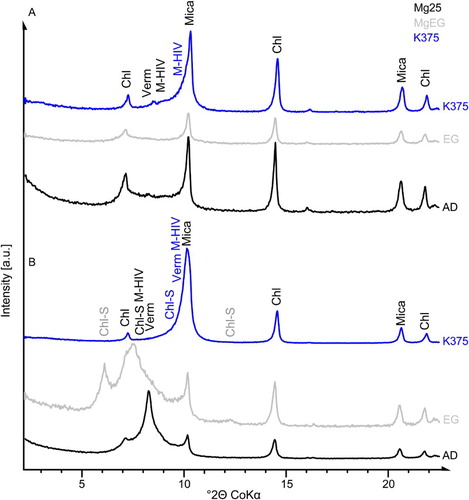
Table 1. Overview of the clay phases of the parent rock and river sediment material based on bulk XRD patterns.
The dominant phyllosilicates of the river sediment bulk material were found to be mica (dioctahedral and trioctahedral; Rieder et al. Citation1998; ), trioctahedral chlorite (medium-Fe to Fe-rich clinochlore; B) and di- or trioctahedral vermiculite (). Dioctahedral vermiculite is a typical weathering product of mica and chlorite, and is more common than trioctahedral vermiculite in soil (Schulze Citation1989). Vermiculite interlayers were predominately occupied by divalent cations such as Mg (two water layers), as indicated by the c. 14.2 Å reflection in the AD condition, but minor amounts of monovalent occupied vermiculite, as indicated by the c. 12.4 Å reflection in the AD condition, were also identified. Because vermiculite was almost absent in the parent material, an abundance of vermiculite in the soils indicates formation by weathering.
The <2 µm fraction contained significantly higher amounts of at least two interstratifications with a strong reflection at 12.3 Å and a broad shoulder towards lower d-values (AD). Upon EG solvation (B), the strong reflection at 12.3 Å split into a narrow reflection at 13.5 Å and a broader one at 16.6 Å, both indicating expandable layers. The layers of both phases collapsed to 10 Å during heat treatment up to 375 °C under vacuum conditions after K‐saturation (B), and a pronounced shoulder remained at the low angle side. The phase with d(EG) = 13.5 Å was interpreted as a mica-vermiculite (M(0.40)-V) interstratification, and the phase with d(EG) = 16.6 Å (and with d = 8.3 Å) as a chlorite-smectite (Chl(0.15)-S) interstratification. These interstratifications presumably originated from biotite and chlorite, respectively. The di- or trioctahedral character of both interstratifications could not be identified in this mixture. Discrete mica (dioctahedral and trioctahedral) and chlorite were present, similar to the bulk material, but in lower concentrations. Kaolinite was not found in the river sediment using XRD.
The presence of M-V and Chl-S interstratifications next to chlorite and tri- and dioctahedral micas indicated that both the parent material and the river sediment were partly acidic weathered. However, the parent material containing lower amounts of interstratifications and higher amounts of carbonates was less affected by weathering than the river sediment with an absence of carbonates and higher amounts of HIM interstratifications. These weathering conditions rapidly produced expandable clay minerals, which were observed in the XRD patterns after EG solvation (B).
Mineralogical composition of soil samples
Bulk samples
The non-phyllosilicates of the chronosequence were similar to the parent material with a predominance of quartz, Na-rich feldspar (± albite) and a clinopyroxene group mineral. Ilmenite and Fe oxides such as hematite, magnetite and maghemite were also present. The absence of carbonates in almost all of the chronosequence samples (except for a 0.5 kyr topsoil and a 60 kyr subsoil B sample) indicated acidic weathering of the parent rock.
General features of clay fractions (<2 µm)
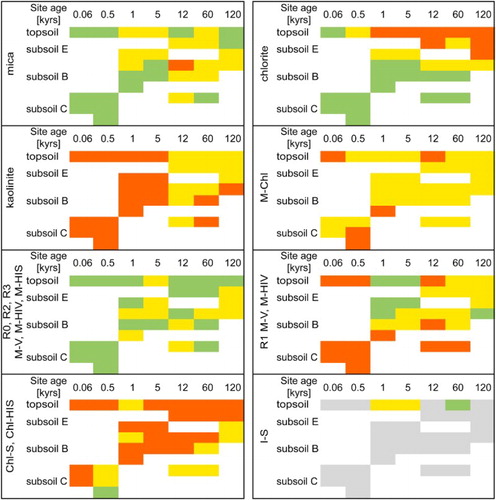
The clay mineral composition of the chronosequence soil samples was dominated by chlorite (primarily trioctahedral with varying Fe contents), mica (both, di- and trioctahedral) and several interstratifications (see below; A). However, the amount of chlorite generally decreased with increasing sample age and was completely absent from older topsoils (≥1 kyr), and intensively weathered soils (120 kyr), which indicated progressive weathering (). These results are consistent with reports by Mokma et al. (Citation1973) who described low chlorite content in topsoils due to weathering of chlorite, but higher chlorite contents in subsoils B due to chloritisation of vermiculite. Mica is first depleted (0.06–12 kyr) due to the transformation of biotite to vermiculite and interstratifications that cause decreasing mica reflections. These interstratifications are then weathered, leading to an apparent enrichment of dioctahedral mica such as muscovite at sites >12 kyr, which is more stable to weathering ().
Figure 5. XRD patterns of the preferred oriented <2 µm fractions. A, A 0.5 kyr subsoil C sample showing M-HIV interstratifications as shoulders. B, The 5 kyr topsoil horizon where M-HIV interstratifications (showing a tendency towards ordering (R1) as indicated by an almost rational basal series) and I-S occur. C, The 0.06 kyr and 0.5 kyr topsoil samples showing a M-HIV interstratification, and in case of the 0.5 kyr A horizon, additional M-Chl interstratification (marked with an arrow at 4.85 Å in the 0.5 kyr A horizon only); changes upon EG solvation are due to expandable HIV layers in M-HIV. D, A 0.5 kyr subsoil C sample showing Chl-S reflections with low full-width at half-maximum values. M-HIV, mica-hydroxy-interlayered vermiculite; I-S, illite-smectite; Chl, chlorite; M-Chl, mica-chlorite; Qtz, quartz; Chl-S, chlorite-smectite; Fsp, feldspars.
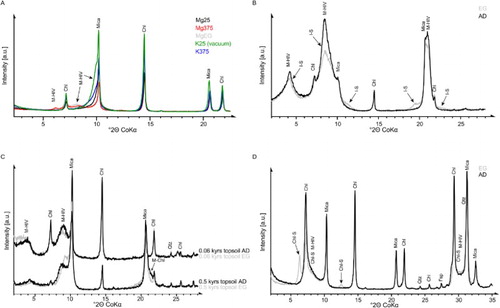
Figure 6. Transformations of clay minerals in soil profiles (<2 µm fractions) over time, based on XRD. Soil horizons were grouped as a function of depth, as described in . The green colour indicates high-intensity reflections, the yellow colour indicates weak intensities and the red colour indicates traces or absence of the phase. I-S interstratifications were introduced by loess depositions and were not weathering products and thus are marked in grey. R1 M-V and R1 M-HIV include hydrobiotite and HI-hydrobiotite.
Chlorite and mica were exclusively present in the >2 µm fraction (e.g. mica and chlorite in 12 kyr subsoil B, or chlorite in 1 kyr topsoil), but were also present in the <2 µm fraction (e.g. mica in 12 kyr topsoil, or chlorite in 12 kyr subsoil B). These results confirm earlier conclusions that chlorite occurs both in coarser fractions and as a weathering product in the <2 µm fraction, especially in deeper horizons of older soils as neoformation (secondary chlorite; Mokma et al. Citation1973). Remnants of primary chlorite and newly formed ‘secondary’ chlorite are difficult to distinguish by XRD. Kaolinite and interstratifications were, if present, enriched in the <2 µm fraction indicating small particles due to neoformation by weathering (see below).
Abundance of kaolinite/halloysite in clay fractions
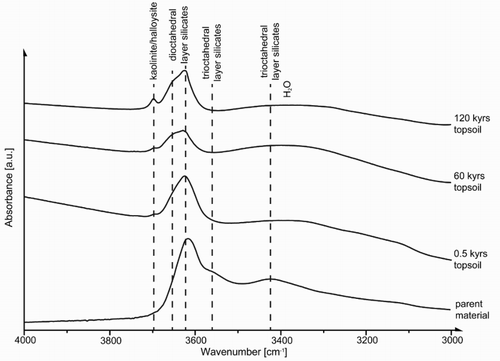
Based on FTIR spectroscopy, kaolinite/halloysite was present in small quantities in all samples (≤1% w/w; ) (Madejová et al. Citation2002; Kaufhold et al. Citation2012). However, according to XRD, kaolinite occurred in older samples ≥12 kyr with a maximum content of c. 5% w/w at 120 kyr (). Using SEM–EDS, kaolinite was found to be fine grained (<1 µm) and associated with organic matter (data not shown). Because of its absence in parent material, kaolinite is considered a newly formed weathering product of primary minerals such as chlorite (Wilson Citation2004) and feldspar promoted by intensive desilification.
Abundance of vermiculite in clay fractions
Vermiculite was present in the >2 µm fractions and occurred in the 0.06 kyr samples (all horizons), in the B and C horizons at 0.5 kyr, in subsoil samples at 1 kyr, and as traces in deeper horizons of some older samples. The selective presence of vermiculite suggests that it was formed from biotite by vermiculitisation during the early stages of soil development, but easily weathered tochlorite or interstratifications with time (Mokma et al. Citation1973). The di- or trioctahedral characteristics of vermiculite could not be identified because of a strong peak overlapping in the die (060) region. HCl treatment (after Wilson Citation2013) resulted in the dissolution of vermiculite, as detected by XRD, indicating either trioctahedral vermiculite with high amounts of Mg and/or Fe3+, or dioctahedral vermiculite originating from weathering of biotite or trioctahedral chlorite (Ristori et al. Citation1974).
Abundance of interstratifications within clay fractions
A wide spectrum of interstratifications was identified in the soils studied, including well and poorly ordered interstratifications, with some containing expandable layers as indicated by EG solvation (A). These phases were characterised by variable amounts of HI, identified upon their thermal reactions after K-intercalation. However, some of the interstratified phases were quite similar. For example, vermiculitic layers with a high degree of HI may have reacted in a similar way to chloritic layers upon the treatments used for identification due to lower expandability (Janssen et al. Citation1997), and low charged vermiculitic layers might be similar to smectitic layers (Moore & Reynolds Citation1997). Because transitions between several types of interstratifications result in similar XRD patterns, some of these interstratifications were grouped (). As described above for vermiculite, the di- or trioctahedral character of these phases could not be identified. However, the amount of trioctahedral phases generally decreased with increasing weathering, indicating that most newly formed phases, such as discrete vermiculite and interstratifications, were dioctahedral ().
All samples showed a broad reflection between 12 and 14 Å (and 3.4–3.5 Å) in the AD condition (Mg saturation). Upon EG solvation, expandable layers were detected that partially collapsed during heat treatment (K375). The resulting broad reflection between 10 and 12 Å indicates a random or short-range ordered M-HIV interstratification (A) with a micaceous layer content varying between 40% and 70%. The M-V in the 0.06 kyr samples was dominated by micaceous layers, indicating its formation from biotite within only few decades. The degree of HI varied randomly in relation to the site age and sampling depth of the soils. Depending on the degree of HI, the broad peaks >10 Å disappeared and collapsed to a narrow 10 Å peak in the case of a low degree of HI. Increasing HI content led to an incomplete collapse of the vermiculitic layers, resulting in higher d-values. The higher full-width at half-maximum values of those peaks indicated a larger variety of the degree of HI.
Because of the diversity of M-V interstratifications and the specialty of ordered interstratifications such as R1 M-V or hydrobiotite, both groups were listed separately (). Unlike chlorite and kaolinite, no distinct trend was observed regarding the occurrence of the disordered M-V phases. However, ordered M-V (R1 M-V and hydrobiotite) was present only in samples older than 0.5 kyr (B); the amount increased with site age and decreased with soil depth, in contrast to the occurrence of mica. This suggests that formation was driven by hydrolytic weathering of biotite (Farmer & Wilson Citation1970). The existence of regularly interstratified M-V and hydrobiotite as a weathering product from biotite was described by Mokma et al. (Citation1973) and Wilson (Citation2004) for A horizons of older soils of the Franz Josef Glacier chronosequence (>12 kyr), and for samples from Scotland (Bain et al. Citation1990). According to Mokma et al. (Citation1973), ordered M-V developed within 1 kyr of soil development. However, based on XRD results, the ordered interstratifications present in these samples were not assumed to be hydrobiotite, because of very different full-width at half-maximum values of the (00 l) series (see Bailey Citation1982; Guggenheim et al. Citation2006).
Most of the samples showed a broad reflection between 4.8 and 4.9 Å that did not change upon EG solvation (C), and K-saturation. This phase was interpreted as mica-chlorite interstratification (M-Chl) with micaceous layer contents of between 45% and 70%. It occurred randomly in relation to site age or horizon depth of the samples. M-Chl showed reflections similar to that of M-HIV, but could be distinguished by observation of the 4.7–5.0 Å reflection (Sawhney Citation1989; Moore & Reynolds Citation1997).
As well as clay minerals present in most soil samples, there were features present in only some samples that occurred independent of site age, soil depth or type of pedogenetic horizon. For example, some of the samples showed a broad reflection between 8.01 and 8.36 Å that disappeared upon EG solvation, whereas a 16.0–16.6 Å peak strongly increased (D). This structure partially collapsed upon K-saturation and/or heating, indicating expandable layers. This type of interstratification was interpreted as chlorite-smectite (Chl-S) with varying degrees of HI (Chl-HIS) and occurred randomly in relation to the site ages, but preferentially in samples of greater depth. This suggests that Chl-S was a metastable weathering product formed in the acid topsoil. The amount of chloritic layers in this type of interstratification was estimated to range from 10% to 35%.
Some samples contained vermiculite (one water layer) that occurred randomly, but mainly in the <2 µm fractions. Dioctahedral illite-smectite interstratifications (I-S) without HI (B) have been recognised only for three topsoil samples at 1, 5 and 60 kyr, all of them showing signs of leaching processes (AE horizons). The amount of illitic layers within this interstratification varied between 50% and 70%. This dioctahedral I-S was assumed to be neither primarily present nor originated by weathering in situ, but introduced by loess depositions (Almond et al. Citation2001). This was supported by the fact that the highest I-S content was found in the 60 kyr AE sample, a site which received significant loess depositions 24–50 kyr ago (Almond et al. Citation2001). However, the presence of I-S traces in the topsoil of the 1 and 5 kyr samples cannot be explained.
The occurrence of chlorite-vermiculite interstratifications (Chl-V) could not be confirmed due to overlapping reflections caused by the interstratifications described above, especially with discrete chlorite and mica. Whether minor amounts of this type of interstratification are additionally present in these samples could not be verified, however, Chl-V is a common weathering product of chlorite in soils (Wilson Citation2013).
Categories of interstratified clay minerals depending on expandability
The broad peak located at c. 23–27 Å (AD) indicates the presence of interstratifications (B). In this study, interstratifications with different amounts of expandable layers were observed, with most interstratifications containing low amounts of expandable layers and being dominated by micaceous or chloritic layers, or high degrees of HI. On the one hand, in less-weathered samples, this is due to low amounts of vermiculitic and smectitic layers that evolved during weathering by K-depletion of biotite and chlorite degradation (e.g. subsoil B of 1 kyr). On the other hand, in more intensively weathered samples, this is due to an increasing amount of HI, which is accompanied by a progressive loss of expandability (Janssen et al. Citation1997), initiated by the dissolution of Al from chlorite and feldspar. This interpretation correlates with the occurrence of biotite and chlorite, which are assumed to be the main sources of the observed interstratifications. However, after EG solvation, some samples showed a significant shift of the c. 23–27 Å peak (AD) up to +1.7 Å, indicating the presence of interstratifications with high amounts of expandable layers such as I-S or Chl-S, without or with low amounts of HI. These include topsoil horizons with high amounts of I-S (; e.g. topsoils of 1 or 60 kyr) or subsoil C samples of 0.5 and 60 kyr that contained higher amounts of Chl-S. Most subsoil B and C samples are considered to be only slightly weathered, as indicated by a higher amount of intrinsically non-expandable layers (e.g. micaceous or chloritic layers). By contrast, topsoil and subsoil E horizons are assumed to be more intensively weathered resulting in higher amounts of expandable or a higher amount of HI layers, because these samples are close to the soil surface.
Appropriate Rietveld models to allow quantification of the amounts of each interstratification and HI to be quantified were not available. Because transitions between the interstratifications are possibly due to vermiculitisation, chloritisation and HI, soils may exhibit a varying degree of expandable interstratifications with time depending on weathering conditions (precipitation and pH), thus favouring both the destruction and neo-formation of clay mineral phases, respectively.
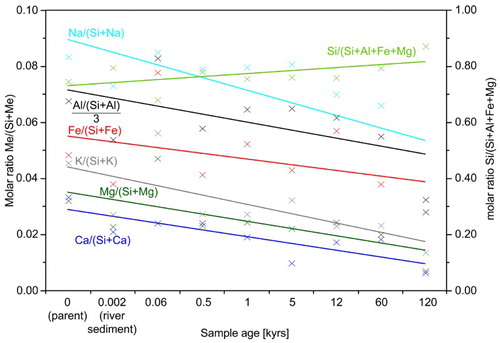
Acidity as major driver of weathering and clay transformation
Soil pH values ranged between 4.1 and 6.4, and generally decreased over time, particularly in topsoil horizons (Turner et al. Citation2014; ). The soil reaction in the organic layers was fairly constant across the gradient (4.1 ± 0.3; not shown), suggesting that differences in aboveground biomass or plant species were less important for pH variations in the mineral soil. Input of acids into topsoils over time, in addition to ongoing silicate weathering (Burke et al. Citation2007) thus enhance the exchangeable soil acidity in the older topsoils. Concurrently, XRF-derived elements Al, Fe, Mg, Ca, Na and K were found to decrease with increasing sample age, indicating a depletion of these elements relative to Si (). Typically, trace elements such as Ti are used for normalisation, although this was not possible due to its occurrence in easily weatherable biotites. Instead, normalisation was performed using Si. On the one hand, Si is present in weatherable minerals of the soils studied; on the other hand, Si is present in more chemically stable minerals in this environment such as quartz. By contrast, Al or Fe cannot be used because these elements were depleted in the soils studied (). The value determined for every site age is the average of element concentrations with respect to bulk densities and the thicknesses of all horizons present. The enrichment of Si relative to structural elements that are incorporated in the tetrahedral and/or octahedral positions of silicates (Al, Fe, Mg), suggests loss of these elements by weathering of biotites, chlorites and feldspars, while stable quartz became relatively enriched (according to Mokma et al. Citation1973). However, Egli et al. (Citation2001) reported for proglacial Gletsch samples (Switzerland), that Si is also depleted, especially in topsoils, which might also be the case here. Based on the pH values (between 4.7 and 6.4) and XRD results (chlorite still present, but lowest amounts of intermediate weathering products), clay fractions of 0.06 and 0.5 kyr soils reflect a low degree of weathering (). For example, the pH of the most unweathered sample (0.06 kyr C) was only slightly acidic (pH 6.4). The intermediate aged samples (1–60 kyr) represented a medium degree of weathering, as indicated by pH values between 4.1 and 5.5, and a higher diversity of intermediate weathering products (several interstratifications and vermiculite). The 120 kyr samples, by contrast, represented the most advance degree of weathering, as revealed by a higher amount of kaolinite despite pH values similar to the intermediate aged samples.
Figure 8. Soil pH values measured in water, reflecting the degree of weathering. Soil horizons were grouped as a function of depth into (a) pure and transitional A horizons (topsoil), (b) horizons showing bleached zones indicative of water and acid leaching (subsoil E), and (c) horizons enriched in pedogenic Fe and Al oxides (subsoil B), and deeper horizons from which the soil developed (subsoil C). The green colour indicates high pH values, the yellow colour indicates medium pH values, and the red colour low pH values (acidic). All values taken from Turner et al. (Citation2014).
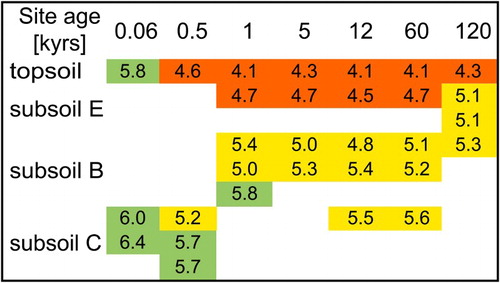
Figure 9. Average XRF-derived molar element ratios of soils referred to horizon thickness and bulk density, illustrating the depletion of elements relative to Si. Si is enriched relative to other structure forming elements. Ratios were derived from mean values calculated from each soil horizon at a given site.
Summary and conclusions
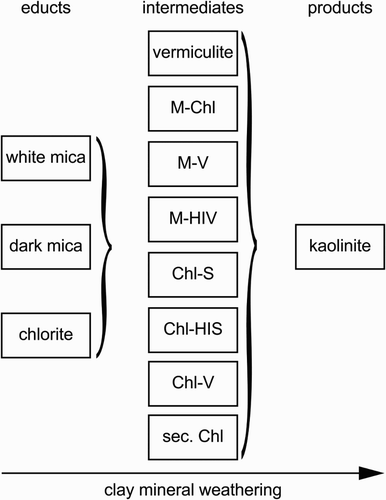
Soil samples from the Franz Josef Glacier chronosequence, New Zealand, were found to be complex mixtures of clay minerals, especially interstratifications. In addition to discrete clay minerals such as chlorite, micas, vermiculite and kaolinite, five types of interstratifications were detected. Quantification of XRD patterns was not possible due to the complex mineralogy and the lack of structural models necessary for the Rietveld method (see Ufer et al. Citation2012a, Citation2012b for I-S interstratifications and Ufer and Kleeberg Citation2015, for combined Rietveld refinement of different XRD pattern). Based on the results presented here, weathering can be described as a process starting as soon as ice coverage disappeared, as shown for the slightly weathered parent material and the river sediment. Clay mineral interstratifications were found to act as intermediate phases resulting from the weathering of educts, but were weathered themselves, forming products such as kaolinite (). Because of the high annual precipitation and increasing soil acidity, less chemically stable minerals such as trioctahedral mica and chlorite, or feldspar (educts) disappeared within 5 kyr upon Al release, and transformed into transitional phases, such as vermiculite or interstratifications with varying degrees of ordering and amounts of HI. Released elements partly recombined into new mineral phases such as goethite, hematite or maghemite, or phases that were amorphous to X‐rays (in the case of Fe and Al), or dissolved and were trapped as polymers by existing mineral phases such as vermiculite or vermiculitic layers of interstratifications (in the case of Al). The latter case led to the formation of HI phases. Further weathering under these perhumic conditions resulted in the formation of kaolinite and Al phases such as gibbsite and amorphous products. Future studies should focus on a Rietveld-based description of the interstratifications; however, to date, no structural models are available except for I-S interstratifications. Such models would allow the quantification of individual phases and X‐ray amorphous materials, including organic matter, to better understand the mass balance of clay transformation processes as well as to relate the clay mineral abundance to soil functioning, e.g. organic matter accumulation and stabilisation. The influence of organic matter on the process of HI seems to be interesting because much of this organic matter is held by secondary soil minerals such as Fe and Al oxyhydroxides and oxides as well as clay minerals (Kleber et al. Citation2015).
Table S2
Download MS Word (138.5 KB)Figure S3
Download PNG Image (3 MB)Document S2
Download MS Word (34 KB)Table S1
Download MS Word (36.5 KB)Acknowledgements
We kindly acknowledge the support of Sarah Richardson and Duane Peltzer from Landcare Research, Lincoln, New Zealand, as well as the Department of Conservation at Franz Josef Glacier.
Associate Editor: Dr Richard Wysoczanski.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Almond PC, Moar NT, Lian OB. 2001. Reinterpretation of the glacial chronology of South Westland, New Zealand. New Zealand Journal of Geology and Geophysics. 44:1–15. doi: 10.1080/00288306.2001.9514917
- Bailey SW. 1982. Nomenclature for regular interstratifications. American Mineralogist. 67:394–398.
- Bain DC, Mellor A, Wilson MJ. 1990. Nature and origin of an aluminous vermiculitic weathering product in acid Soils from upland catchments in Scotland. Clay Minerals. 25:467–475. doi: 10.1180/claymin.1990.025.4.05
- Banfield JF, Eggleton RA. 1988. Transmission electron microscope study of biotite weathering. Clays and Clay Minerals. 36:47–60. doi: 10.1346/CCMN.1988.0360107
- Barnhisel RI, Bertsch PM. 1989. Chlorites and hydroxy-interlayered vermiculite and smectite. In: Dixon JB, Weed SB, editors. Minerals in soil environments. Madison (WI): Soil Science Society of America; p. 729–788.
- Brigatti MF, Galán E, Theng BKG. 2013. Structure and mineralogy of clay minerals. In: Bergaya F, Lagaly G, editors. Handbook of clay science. Amsterdam: Elsevier; p. 21–81.
- Brindley GW, Brown G. 1984. Crystal structures of clay minerals and their X-ray identification. London: Mineralogical Society Monograph No. 5; p. 349–356.
- Burke BJ, Heimsrath AM, White AF. 2007. Coupling chemical weathering with soil production across soil-mantled landscapes. Earth Surface Processes and Landforms. 32:853–873. doi: 10.1002/esp.1443
- Coleman NT, LeRoux FH, Cady JG. 1963. Biotite - Hydrobiotite - Vermiculite in Soils. Nature. 198:409–410. doi: 10.1038/198409c0
- Dohrmann R, Rüping KB, Kleber M, Ufer K, Jahn R. 2009. Variation of preferred orientation in oriented clay mounts as a result of sample preparation and composition. Clays and Clay Minerals. 57:686–694. doi: 10.1346/CCMN.2009.0570602
- Egli M, Fitze P, Mirabella A. 2001. Weathering and evolution of soils formed on granitic, glacial deposits: results from chronosequences of Swiss alpine environments. Catena. 45:19–47. doi: 10.1016/S0341-8162(01)00138-2
- Farmer VC, Wilson MJ. 1970. Experimental conversion of biotite to hydrobiotite. Nature. 226:841–842. doi: 10.1038/226841b0
- Grapes R, Otsuki M. 1983. Peristerite compositions in quartzofeldspathic schists, Franz Josef-Fox Glacier Area, New Zealand. Journal of Metamorphic Geology. 1:47–61. doi: 10.1111/j.1525-1314.1983.tb00264.x
- Grindley GW. 1963. Structure of the alpine schists of South Westland, Southern Alps, New Zealand. New Zealand Journal of Geology and Geophysics. 6:872–930. doi: 10.1080/00288306.1963.10423630
- Guggenheim S, Adams JM, Bain DC, Bergaya F, Brigatti MF, Drits VA, Formoso MLL, Galán E, Kogure T, Stanjek H. 2006. Summary of recommendations of nomenclature committees relevant to clay mineralogy: report of the Association Internationale pour l’Etude des Argiles (AIPEA) Nomenclature Committee for 2006. Clay Minerals. 41:863–877. doi: 10.1180/0009855064140225
- Hillier S. 2001. Particulate composition and origin of suspended sediment in the R. Don, Aberdeenshire, UK. Science of The Total Environment. 265:281–293. doi: 10.1016/S0048-9697(00)00664-1
- Ismail FT. 1969. Role of ferrous iron oxidation in the alteration of biotite and its effect on the type of clay minerals formed in soils of arid and humid regions. American Mineralogist. 54:1460–1466.
- Ismail FT. 1970. Biotite weathering and clay formation in arid and humid regions, California. Soil Science. 109:257–261. doi: 10.1097/00010694-197004000-00011
- Jagodzinski H. 1949. Eindimensionale Fehlordnung in Kristallen und ihr Einfluss auf die Röntgeninterferenzen. I. Berechnung des Fehlordnungsgrades aus den Röntgenintensitäten. Acta Crystallographica. 2:201–207. doi: 10.1107/S0365110X49000552
- Janssen RPT, Bruggenwert MGM, van Riemsdijk WH. 1997. Interactions between citrate and montmorillonite-Al hydroxide polymer systems. European Journal of Soil Science. 48:463–472. doi: 10.1046/j.1365-2389.1997.00097.x
- Kaufhold S, Hein M, Dohrmann R, Ufer K. 2012. Quantification of the mineralogical composition of clays using FTIR spectroscopy. Vibrational Spectroscopy. 59:29–39. doi: 10.1016/j.vibspec.2011.12.012
- Kleber M, Eusterhues K, Keiluweit M, Mikutta C, Mikutta R, Nico PS. 2015. Chapter one – mineral-organic associations: formation, properties, and relevance in soil environments. In: Sparks DL, editor. Advances in agronomy. Academic Press. Vol. 130; p. 1–140. doi:10.1016/bs.agron.2014.10.005.
- Lanson B, Ferrage E, Hubert F, Pret D, Mareschal L, Turpault M‐P, Ranger J. 2015. Experimental aluminization of vermiculite interlayers: an X-ray diffraction perspective on crystal chemistry and structural mechanisms. Geoderma. 249–250:28–39. doi: 10.1016/j.geoderma.2015.03.005
- Larson IJ, Almond PC, Eger A, Stone JO, Montgomery DR, Malcolm B. 2014. Rapid soil production and weathering in the Southern Alps, New Zealand. Science. 343:637–640. doi: 10.1126/science.1244908
- Madejová J, Kečkéš J, Pálková H, Komadel P. 2002. Identification of components in smectite/kaolinite mixtures. Clay Minerals. 37:377–388. doi: 10.1180/0009855023720042
- Mehra OP, Jackson ML. 1960. Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clays and Clay Minerals. 7:317–327. doi: 10.1346/CCMN.1958.0070122
- Méring PJ. 1949. L’interférence des rayons X dans les systèmes à stratification désordonée. Acta Crystallographica. 2:371–377. doi: 10.1107/S0365110X49000977
- Meunier A. 2007. Soil hydroxy-interlayered minerals: a re-interpretation of their crystallochemical properties. Clays and Clay Minerals. 55:380–388. doi: 10.1346/CCMN.2007.0550406
- MacEwan DMC. 1954. “Cardenite,” a trioctahedral montmorillonoid derived from biotite. Clay Minerals. 2:120–126. doi: 10.1180/claymin.1954.002.11.07
- Mikutta R, Kleber M, Kaiser K, Jahn R. 2005. Review: organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate. Soil Science Society of America Journal. 69:120–135. doi: 10.2136/sssaj2005.0120
- Mokma DL, Jackson ML, Syers JK, Gibbons FR. 1973. Mineralogy of a chronosequence of soils derived from greywacke and mica-schist alluvium from Westland, New Zealand. New Zealand Journal of Science. 16:769–797.
- Moore DM, Reynolds RC Jr. 1997. X-Ray diffraction and the identification and analysis of clay minerals. New York (NY): Oxford University Press. p. 378.
- Rich CI. 1968. Hydroxy interlayers in expansible layer silicates. Clays and Clay Minerals. 16:15–30. doi: 10.1346/CCMN.1968.0160104
- Richardson SJ, Peltzer DA, Allen RB, McGlone MS, Parfitt RL. 2004. Rapid development of phosphorus limitation in temperate rainforest along the Franz Josef soil chronosequence. Oecologia. 139:267–276. doi: 10.1007/s00442-004-1501-y
- Rieder M, Cavazzini G, D´Yakonov YS, Frank-Kamenetskii VA, Gottardi G, Guggenheim S, Koval PV, Mueller G, Neiva AMR, Radoslovich EW, et al. 1998. Nomenclature of micas. The Canadian Mineralogist. 36:41–48.
- Ristori GG, Cecconi S, Vidrich V, Pacifici G. 1974. Selective dissolution and formula derivation of clay vermiculite from some Tuscan soils. Clay Minerals. 10:279–287. doi: 10.1180/claymin.1974.010.4.06
- Sawhney BL. 1989. Interstratification in layer silicates. In: Dixon JB, Sawhney BL, editors. Minerals in soil environments. Madison (WI): SSSA Book Series 1; p. 789–828.
- Schulze DG. 1989. An introduction to soil mineralogy. In: Dixon JB, Weed SB, editors. Minerals in soil environments. Madison (WI): Soil Science Society of America; p. 1–34.
- Stevens PR. 1968. A chronosequence of soils near the Franz Josef glacier [PhD thesis]. New Zealand: University of Canterbury. 389 p.
- Sverdrup H, Warfvinge P, Nihlgård B. 1994. Assessment of soil acidification effects on forest growth in Sweden. Water, Air, & Soil Pollution. 78:1–36. doi: 10.1007/BF00475665
- Turner S, Schippers A, Meyer-Stüve S, Guggenberger G, Gentsch N, Dohrmann R, Condron LM, Eger A, Almond PC, Peltzer DA, et al. 2014. Mineralogical impact on long-term patterns of soil nitrogen and phosphorus enzyme activities. Soil Biology and Biochemistry. 68:31–43. doi: 10.1016/j.soilbio.2013.09.016
- Ufer K, Kleeberg R. 2015. Parametric Rietveld refinement of coexisting disordered clay minerals. Clay Minerals. 50:287–295.
- Ufer K, Kleeberg R, Bergmann J, Dohrmann R. 2012a. Rietveld refinement of disordered illite-smectite mixed-layer structures by a recursive algorithm. I: one-dimensional patterns. Clays and Clay Minerals. 60:507–534. doi: 10.1346/CCMN.2012.0600507
- Ufer K, Kleeberg R, Bergmann J, Dohrmann R. 2012b. Rietveld refinement of disordered illite-smectite mixed-layer structures by a recursive algorithm. II: powder-pattern refinement and quantitative phase analysis. Clays and Clay Minerals. 60:535–552. doi: 10.1346/CCMN.2012.0600508
- Vincente MA, Razzaghe M, Robert M. 1977. Formation of aluminium hydroxy vermiculite (intergrade) and smectite from mica under acidic conditions. Clay Minerals. 12:101–112. doi: 10.1180/claymin.1977.012.02.01
- Wilson MJ. 2004. Weathering of the primary rock-forming minerals: processes, products and rates. Clay Minerals. 39:233–266. doi: 10.1180/0009855043930133
- Wilson MJ. 2013. Rock-forming minerals. Volume 3C. Sheet silicates: clay minerals. 2nd ed. London: The Geological Society; p. 724.