 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Whitebait comprise a culturally, commercially and recreationally important fishery in New Zealand, where post-larvae are netted while returning from their marine phase. In this study, we expanded an historical (1964) sampling programme to gain a contemporary understanding of the species composition of the whitebait fishery; 87 rivers were sampled over six months in 2015. Over the entire country, >12 species were found in samples and 87.6% of these were īnanga (Galaxias maculatus). Kōaro (G. brevipinnis) and banded kōkopu (G. fasciatus) were abundant in some rivers and regions at particular times of the year. Buller was the most variable region, spatially and temporally, for species composition; Canterbury was the least variable. Banded kōkopu whitebait migrated one month earlier north of Cook Strait than in the south. There was a positive association between the abundance of kōaro and banded kōkopu in samples and the level of indigenous forest cover in catchments. Compared to samples from 50 years ago, there was a greater proportion of kōaro and banded kōkopu whitebait throughout the country. This spatio-temporal variability requires fishery regulations to be more tailored and flexible if they are to conserve the diversity of life-histories present in the catch and sustain the whitebait fishery.
Introduction
In New Zealand, ‘whitebait’ is the collective term for large shoals of post-larval fish that are caught in a culturally, commercially and recreationally important fishery as they migrate from coastal waters into freshwater. Primarily, these fish come from the Galaxiidae family, but several other families (e.g. Retropinnidae and Anguillidae) have been recorded in catches (McDowall Citation1965). Most diadromous species make their upstream migration in single-species shoals (e.g. shad, smelt and Pacific salmon), but the shoals caught in the whitebait fishery contain a mix of five galaxiid species (McDowall and Eldon Citation1980). Globally, multi-species fisheries are not unusual (see May et al. Citation1979), but the exploitation of post-larvae is very uncommon. Management of the whitebait fishery is further complicated by the diversity of life-histories present in the five galaxiids. The fishery comprises mostly the relatively small, annual species īnanga (Galaxias maculatus) together with much lower proportions of four larger, perennial species: kōaro (G. brevipinnis), banded kōkopu (G. fasciatus), giant kōkopu (G. argenteus) and shortjaw kōkopu (G. postvectis; McDowall Citation1965).
There is increasing concern that the whitebait fishery is declining because of multiple stressors on populations that may have differentially affected target species (Goodman et al. Citation2014) and reduced their ability to sustain the fishery. Populations of most of the perennial whitebait species have suffered severe declines in areas where deforestation (McDowall Citation1990), pasture development (Rowe et al. Citation1999), or wetland drainage (Rowe et al. Citation2000) have occurred. Understanding the species composition of the fishery in different geographic regions is a necessary precursor to delineating the effects of anthropogenic impacts from natural variability. Unfortunately, there is limited information on the current species composition of the whitebait catch, wide- and local-scale variability, or temporal variability during the fishing season.
McDowall (Citation1965) estimated that īnanga make up 85.2% of the whitebait catch, but this was based on ‘quite inadequate’ sampling of rivers in Hawke's Bay, Taranaki and Nelson- Marlborough, and no samples from the Bay of Plenty or Southland, all of which are important whitebaiting regions (Kelly Citation1988). A small proportion of īnanga (5%) probably live two years, but the species is predominantly annual with a single year class dominating most adult populations (Stevens et al. Citation2016). Īnanga population sizes are expected to fluctuate widely because of stochastic environmental processes affecting larval survival and year-class strengths (Ueta et al. Citation1999). Consequently, īnanga populations, and the whitebait fishery, are exposed to sudden and serious declines if a year-class is compromised or fails.
Failure of a year-class in the perennial whitebait species will also potentially reduce population sizes and distort age/size-frequency distributions, but these species can live between eight (kōaro) and thirty (giant kōkopu) years. There is, therefore, some buffering of populations across the multiple year classes that comprise them. Over all the whitebait species, the perennial ones are more resilient to natural variability in larval survival and thereby potentially add some resilience to the fishery based mostly on the annual īnanga. This potential, however, could only be realised if the number of post-larvae of perennial species returning to the coast is sufficient for a fishery. The spatial and temporal returns of these species are unknown over most of New Zealand.
Here, we hypothesise that the species composition of the whitebait catch will vary spatially around New Zealand. This variability is likely to arise from complex coastal currents (Chiswell and Rickard Citation2011) and the non-continuous distribution of populations of the three kōkopu whitebait species (McDowall Citation1990). Given the wide latitudinal spread of New Zealand, temporal offsets in spawning between regions (Taylor Citation2002; Stevens et al. Citation2016) and differing regional larval development rates (Egan Citation2017) it is expected that larvae will enter and leave the marine environment differentially around New Zealand. Consequently, we hypothesise that the species composition of the whitebait catch will vary temporally within and between regions. Finally, given the diminishing abundance of adults of the perennial whitebait species (Hanchet Citation1990; Bonnett Citation2000; McDowall Citation2000; Rowe et al. Citation2000), we hypothesise that the proportions of these species in the whitebait catch will have reduced significantly from those recorded in earlier studies (cf. McDowall Citation1965; Rowe et al. Citation1992).
Methods
Collection and species identification
Whitebait were collected by experienced fishers from 87 rivers throughout New Zealand using conventional netting methods (See Table S1, Supplemental Data). Samples (n = 290) were collected haphazardly from July to December 2015. For 37 rivers, only a single sample was obtained, but most regions (except for Auckland, Coromandel, Taranaki and Wairarapa) had samples taken from at least two rivers on multiple occasions. Sampling sites included important whitebaiting rivers (Kelly Citation1988), a subset of sites from past surveys (e.g. McDowall Citation1965; McDowall and Eldon Citation1980; Rowe et al. Citation1992), and a range of rivers with diverse characteristics. Samples of whitebait were frozen after capture.
Whitebait enter waterways in mixed-species shoals (McDowall and Eldon Citation1980). When possible, multiple shoals were caught before a sample of whitebait was removed from the catch. Previous research had established that this methodology, and a single sample of >100 whitebait, provided a good estimate of a fisher's catch (see Rowe et al. Citation1992). Therefore, only samples containing ≥100 fish were used for all subsequent analyses.
Thawed whitebait were identified using keys developed by McDowall and Eldon (Citation1980) and McDowall (Citation1984). Species were identified with a stereo microscope using morphological features. Only fresh-run (non-pigmented) whitebait were used to avoid any bias from post-recruitment processes (Allibone et al. Citation1999). Previous studies have developed genetic markers to confirm species identification of the five whitebait species (Dijkstra and McDowall Citation1997; Charteris and Ritchie Citation2002). After whitebait were morphologically identified to the lowest practical level, specimens were preserved in vials with 70% ethanol. An initial sub-sample of 51 whitebait of known species identity, whitebait where there was some uncertainty, and unidentified whitebait were tested genetically (Genetic Analysis Service, University of Otago) to confirm species identification (see Supplemental Data).
Statistical analyses
Two-way ANOVA was used to compare the species composition of whitebait samples across regions (random) and months (random). Comparisons were restricted to regions (Waikato, Buller, Canterbury, Westland and Southland) with samples of >100 fish from at least three rivers within each of the three months (Sept, Oct, Nov). Data were tested for homogeneity of variances and arcsine transformed where necessary.
To test how much of the variability in the species composition of whitebait samples was accounted for by environmental variables (catchment size, indigenous forest cover and pasture cover), and to find the subset of variables that best explained this variability, a non-parametric multivariate regression analysis was done using the DISTLM module in PERMANOVA+ (Anderson et al. Citation2008). September, October and November data were analysed separately to minimise temporal effects. Initially, individual predictor variables were analysed separately in marginal tests for potential relationships with species composition data. Variables were then subjected to a stepwise forward-selection procedure (sequential test, R2 selection criteria), where the amount of variability explained by each variable added to the model is conditional on the variables already in the models. P-values for the marginal tests were obtained using 9999 permutations of the normalised predictor data, while conditional tests were made using 9999 permutations of residuals under the reduced model. All tests were based on Euclidean dissimilarities, calculated among species composition observations. All multivariate analyses were done using PRIMER 6 and PERMANOVA+. Univariate analyses were used to investigate the association between environmental variables and the occurrence of individual whitebait species. Pearson correlation coefficients for the association between the proportion of each species in whitebait samples and indigenous forest/pasture cover or catchment area, were calculated separately for each month.
Results
In the first sample of genetically tested fish, the genetic species identifications differed for six fish from morphological species identifications. An individual from the Whakatane River that was suspected of being a kōaro was identified genetically as a shortjaw kōkopu. This confirmed the difficulties, as highlighted by McDowall and Eldon (Citation1980), of distinguishing between shortjaw kōkopu and kōaro whitebait. Three whitebait (out of seven) from North Island rivers that showed mixed characteristics of kōaro and giant kōkopu were confirmed as giant kōkopu after genetic identification. Thirteen whitebait (out of 15) that were thought to be giant kōkopu were confirmed with genetic species identification (the remaining two were identified as kōaro). These results were used to resolve regional differences, and all 30 morphological identifications of a second sample were confirmed genetically as being correct.
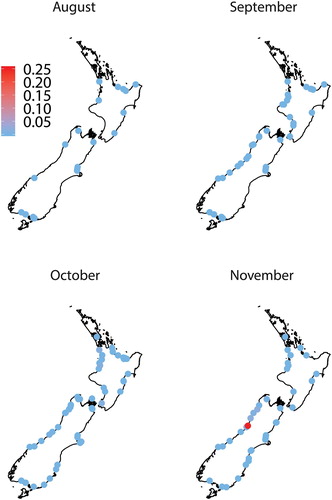
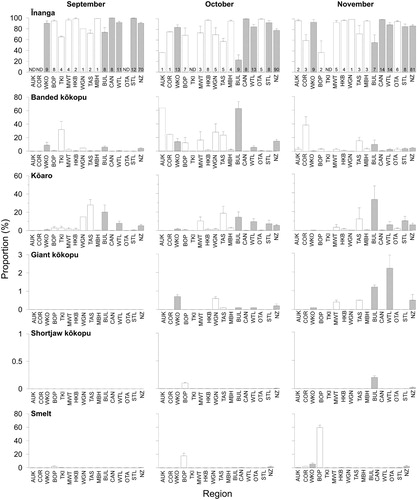
Six whitebait species were found in samples collected throughout New Zealand during the six-month study. ‘Whitebait’ catches also included glass eel and elver stages of eels, juvenile and adult Gobiomorphus spp., freshwater shrimp (Paratya curvirostris), juvenile yellow-eyed mullet (Aldrichetta forsteri), lamprey (Geotria australis) and one juvenile barracouta (Thyrsites atun). Īnanga was the most common species, being found in every region () and all rivers except for a single sample (n = 155) from the Karamea River (Buller) in October that was dominated by banded kōkopu (68%) and kōaro (32%). Banded kōkopu was present in each region, but was absent from some rivers, particularly in Canterbury, Otago and Southland (). Samples from all regions except Auckland and Coromandel contained kōaro (), but this species was also absent from many rivers in Waikato, Bay of Plenty, Hawke's Bay and Canterbury. Giant kōkopu was only found in samples from the west coast of both islands and around Cook Strait (i.e. Tasman-Nelson and Wellington; ). Only four shortjaw kōkopu whitebait were identified and they were found in samples from Bay of Plenty, Buller and Westland. The overall species composition in all samples (n = 290) was 87.6 ± 1.2% ( ± SE) īnanga, 6.9 ± 0.9% banded kōkopu, 4.8 ± 0.7% kōaro, 0.4 ± 0.3% smelt, 0.3 ± 0.1% giant kōkopu and 0.007 ± 0.004% shortjaw kōkopu.
Figure 1. The proportion of īnanga (Galaxias maculatus) in whitebait samples (n ≥ 100 fish) collected over four months from up to 87 rivers around New Zealand.
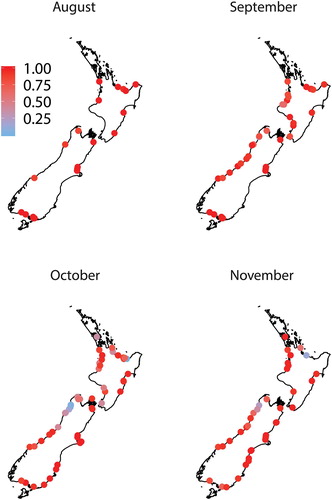
Figure 2. The proportion of banded kōkopu (Galaxias fasciatus) in whitebait samples (n ≥ 100 fish) collected over four months from up to 87 rivers around New Zealand.
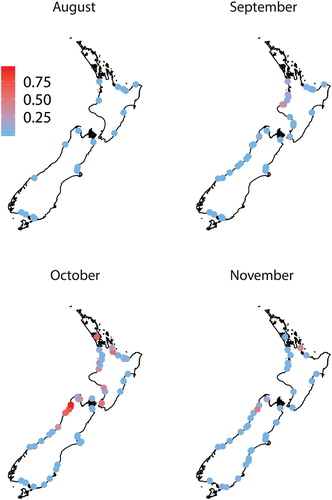
Figure 3. The proportion of kōaro (Galaxias brevipinnis) in whitebait samples (n ≥ 100 fish) collected over four months from up to 87 rivers around New Zealand.
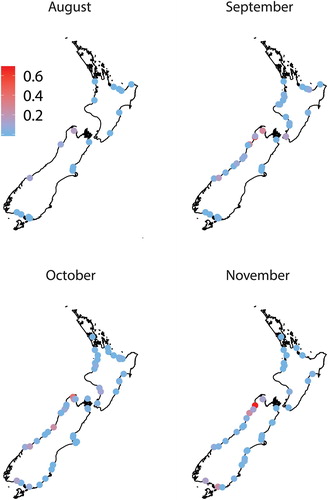
Figure 4. The proportion of giant kōkopu (Galaxias argenteus) in whitebait samples (n ≥ 100 fish) collected over four months from up to 87 rivers around New Zealand.
The proportion of īnanga in whitebait samples was significantly lower in Buller than in Waikato, Canterbury, Westland and Southland (F4,136 = 10.496, P < 0.01, Tukey HSD, P < 0.01; ), particularly in October (F8,136 = 3.962, P < 0.01 Tukey HSD, P < 0.001). The proportion of banded kōkopu in samples did not differ between regions overall (F4,136 = 2.617, P = 0.115; ), but it was higher in Buller and Waikato in October (F8,136 = 13.374, P < 0.001; ). Kōaro was most abundant in Buller samples in all months (F4,136 = 10.442, P < 0.01 Tukey HSD, P < 0.01; ). In most regions, kōaro proportions did not differ between months (F2,136 = 0.762, P = 0.497; ), but in Buller and Southland there was a non-significant trend of more kōaro in later samples (F8,136 = 1.914, P = 0.06; ). Too few samples contained giant kōkopu, shortjaw kōkopu or smelt to test for differences between regions or months ().
Figure 5. The mean (+SE) proportion (%) of the six whitebait species in samples (n ≥ 100 fish) taken over three months (September–November 2015) from 15 regions of New Zealand. The number of samples is shown at the base of bars in the īnanga panels. ND = no data. Regions are: Auckland (AUK), Coromandel (COR), Waikato (WKO), Bay of Plenty (BOP), Taranaki (TKI), Manawatu-Wanganui (MWT), Hawke's Bay (HKB), Wellington (WTN), Tasman-Nelson (TAS), Marlborough (MBH), Buller (BUL), Canterbury (CAN), Westland (WTL), Otago (OTA), Southland (STL) and all of New Zealand (NZ). Grey bars are regions where enough samples were taken in each month to allow comparisons with ANOVA.
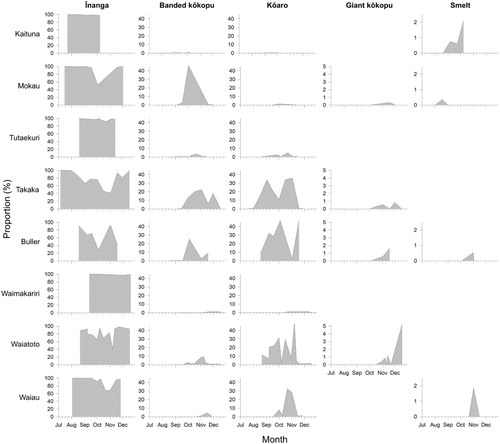
The timing of the influx of some of the less common whitebait species showed a strong latitudinal gradient (). The proportion of banded kōkopu in samples peaked in October in some northern rivers (e.g. Mokau and Tūtaekurī), but they appeared, and peaked, later in southern rivers (e.g. Waiatoto and Waiau). Likewise, kōaro proportions increased greatly in the Takaka and Buller Rivers from early September, but similar increases were not seen in southern rivers (e.g. Waiatoto and Waiau) until early October.
Figure 6. The proportion (%) of five whitebait species in samples (n ≥ 100 fish) taken from eight rivers (plotted from north to south) over up to six months (July–December 2015).
The indigenous forest cover in a catchment was a significant predictor of the species composition of the whitebait captured in that catchment (), but pasture cover and catchment area had little explanatory power. Marginal tests showed that forest cover accounted for 24% of the variation in species composition in September, 16% in October and 9% in November. Sequential tests showed that pasture cover (2–4%) and catchment area (1–4%) added very little to the explained variation after forest cover had already been fitted. In September, October and November the proportion of īnanga in whitebait samples was strongly negatively correlated with a catchment's indigenous forest cover (). Kōaro and banded kōkopu proportions in samples were positively correlated with indigenous forest cover (), but they were also found in very low proportions ( < 3%) in samples from rivers with no indigenous forest cover in their catchment (e.g. Canterbury).
Table 1. Summary of DISTLM models with indigenous forest cover (%) of a catchment as a predictor of the species composition of the whitebait catch. Also shown are Pearson's correlation coefficients between indigenous forest cover (%) and individual species within months.
The combined species composition of whitebait samples from all rivers in New Zealand in 2015 was very similar to that of 50 years ago (). The proportions of īnanga and ‘kōkopu’ whitebait were slightly greater in 2015, with an associated decrease in kōaro. McDowall (Citation1965) did not distinguish between species of ‘kōkopu’ whitebait, but in 2015 the vast majority (97%) of ‘kōkopu’ whitebait were banded kōkopu (). When separated into coarse geographic areas (North Island, South Island east coast and South Island west coast), the proportion of ‘kōkopu’ whitebait had more than doubled in 2015 in each area (). Changes in kōaro proportions were not as extreme or consistent between areas.
Table 2. Mean (±SE) species composition (%) of whitebait samples from all rivers in New Zealand, North Island (both coasts), South Island (east coast) and South Island (west coast) in 1964 (adapted from McDowall Citation1965) and 2015. For 2015 the ‘kōkopu’ species have been separated into banded, giant and shortjaw kōkopu. The number of samples (n) from each area is shown.
The species composition of whitebait samples from four rivers in the Bay of Plenty was markedly different from samples collected in 1983 (). In most rivers, the proportion of īnanga whitebait had increased. The large proportions of kōaro and banded kōkopu seen in earlier samples (Rowe et al. Citation1992) had diminished significantly in all rivers. A single shortjaw kōkopu whitebait was found in a sample from the Whakatane River on 5 October 2015. Smelt made up a large proportion of the catch in the Kaituna River in later months ().
Table 3. Mean (±SE) species composition (%) of whitebait samples from four Bay of Plenty rivers in 1983 (adapted from Rowe et al. Citation1992) and in 2015. The number of samples (n) from each river is shown.
Discussion
This is the first study to capture a simultaneous view of the species composition of the New Zealand whitebait fishery and to incorporate all regions with significant fisheries (e.g. inclusion of Southland, Manawatu-Wanganui and the Bay of Plenty). Nationally, the fishery consisted of very high proportions of īnanga whitebait and lower, and more variable, proportions of non-īnanga whitebait. Banded kōkopu and kōaro whitebait were common in some rivers and at certain times of the year, but giant kōkopu and shortjaw kōkopu were rare and patchily distributed throughout the six-month sampling period.
There were obvious differences in the species composition of whitebait samples from different regions in September, October and November. These differences varied between months, but Buller always had a different species composition to other regions. Whitebait samples from rivers in Buller had higher proportions of non-īnanga species than elsewhere. However, species composition varied considerably among rivers within Buller; southern rivers (e.g. Orowaiti and Buller) generally had higher proportions of īnanga whitebait than northern rivers (e.g. Oparara and Karamea).
The Westland Current forms from water flowing across the Tasman Sea and then moving northeast along the West Coast of the South Island (de Lange et al. Citation2003). Because the Westland Current flows northwards past the mouths of all Buller rivers it is most likely that whitebait entering those rivers are either sourced from Buller or Westland. Australian populations might contribute some īnanga and kōaro whitebait to Buller and Westland rivers (McDowall et al. Citation1998; Waters et al. Citation2000), but whitebait of the three endemic species must come from New Zealand. Coastal currents along the West Coast could move developing larvae northwards, but it is highly unlikely that non-īnanga whitebait entering West Coast rivers are sourced from elsewhere in New Zealand. This suggests that they must be retained along the West Coast for the 4–5 months that they are developing as larvae (McDowall and Kelly Citation1999).
East coast regions, particularly Canterbury, had very low proportions of kōaro and the kōkopu whitebait species. The NZ Freshwater Fish Database shows very limited observations of adult kōkopu in these regions. The Southland Current transports water from the lower West Coast and Southland (where large populations of non-īnanga adults have been observed) up the east coast of the South Island. The scarcity of non-īnanga whitebait along the east coast suggests that the larvae of non-īnanga species may have better retention near their natal streams. Previous work using genetics and otolith microchemistry suggested that kōaro may be resistant to dispersal (Augspurger Citation2017). Otolith microchemistry suggests that īnanga whitebait on the South Island's east coast could be sourced from local rivers or swept from the West Coast and Southland by the Southland or D’Urville Currents (Hickford and Schiel Citation2016).
The species composition of whitebait samples was found to change from July to December with variability among regions, among rivers within regions, and within rivers. Samples from rivers in Hawke's Bay, Marlborough, Canterbury and Otago (all eastern regions) always had large proportions of īnanga whitebait, but samples from Waikato, Taranaki, Manawatu-Wanganui, Tasman-Nelson, Buller, Westland and Southland (mainly western regions) had high proportions of non-īnanga whitebait at some stage during the six month sampling period.
The species composition of whitebait samples often differed between rivers in the same region. In Tasman-Nelson, Buller and Westland, whitebait samples from some rivers had higher proportions of kōaro or banded kōkopu. Generally, the rivers with larger proportions of kōaro whitebait were very large river systems (e.g. Takaka, Buller and Waiatoto Rivers) while the rivers with large proportions of banded kōkopu whitebait were smaller catchments with high forest cover (e.g. Wainui Stream and Waimea Creek). This supports earlier work that suggests active river selection by these whitebait species. Banded kōkopu (Baker and Montgomery Citation2001) and kōaro (Baker and Hicks Citation2003) whitebait exhibit a species-specific attraction to water-borne odours released by adult conspecifics. The odour of adults inhabiting tributary streams may be used to identify rivers containing areas of accessible habitat. McDowall (Citation1965) suggested that the temperature of river water might have a controlling function on the types of whitebait entering the river. Specifically, that cold rivers derived from glaciers and mountain regions are the ones into which kōaro whitebait mostly migrate, whereas ‘kōkopu’ whitebait migrate more abundantly into warmer, forested rivers.
The timing of migration varied among the whitebait species in different regions of New Zealand. Banded, giant and shortjaw kōkopu whitebait appeared earlier in the North Island than in the South Island. Rowe et al. (Citation1992) found peak migrations of banded kōkopu in mid-September in the Bay of Plenty (compared to mid-October in our study) and McDowall and Eldon (Citation1980) observed peak migrations in mid-November in Westland (as did we). The first migration of giant kōkopu whitebait occurred during late September/early October in North Island west coast rivers, from mid-October in Tasman-Nelson, and from early November in Buller and Westland. These timings were consistent with those observed by McDowall (Citation1999), who found giant kōkopu whitebait from November onwards in Westland rivers, and Stancliff et al. (Citation1988) who found pigmented giant kōkopu juveniles migrating in the Waikato River from mid-October. The earlier migration of banded, giant and shortjaw kōkopu whitebait in the North Island may be due to latitudinal differences in the temperature cues for the onset of spawning (Taylor Citation2002; Stevens et al. Citation2016) and the shorter larval duration of whitebait in the north (Rowe and Kelly Citation2009; McClintock Citation2018).
The West Coast whitebait fishery is currently managed separately from the rest of New Zealand with additional rules, a reduced fishing season and closed rivers. For decades, the West Coast whitebait fishery has had special provisions because it is thought to be distinctive and highly productive. Our study shows that other important whitebaiting regions are as diverse and distinctive in species composition as the West Coast. In particular, we found that Waikato, Bay of Plenty, Manawatu-Wanganui, Wellington and Tasman-Nelson also have high proportions of non-īnanga whitebait species including kōaro, banded and giant kōkopu.
In 1994, the West Coast whitebait fishery was shortened by two weeks for conservation of the later-migrating giant kōkopu whitebait. In our study, giant kōkopu (and shortjaw kōkopu) whitebait were found in samples from West Coast rivers in late October/early November when whitebaiting is still allowed. In the North Island, giant kōkopu whitebait were found throughout October and November during the open fishing season. If fishery managers want to allow for greater escapement of giant kōkopu whitebait, then the open fishing season should be further shortened on the West Coast and possibly in other regions.
We expected that whitebait samples collected in 2015 would contain higher proportions of īnanga than samples taken 50 years earlier because īnanga are habitat generalists and more tolerant of lower water quality associated with changes in land use than kōaro or kōkopu species (Boubée et al. Citation1997; Urbina et al. Citation2011). However, the opposite occurred, with higher proportions of kōaro and kōkopu species found in samples from the North Island and the east and west coasts of the South Island in 2015 than in 1964. While this change might be due to īnanga populations being negatively impacted by land use intensification (particularly dairying) in their lowland habitat (Baskaran et al. Citation2009), it is more likely due to sampling bias. The dates of sampling in 1964 are not known and there may have been a greater number of samples collected in 2015 at a time when more non-īnanga whitebait were migrating (e.g. October and November). Furthermore, McDowall (Citation1965) included all whitebait samples with >9 fish in his analysis. In our analysis, small samples (<100 fish) were not included in any analyses because Rowe et al. (Citation1992) found marked differences in species composition between small and large samples.
The Kaituna River was sampled extensively in 1983 and 2015 and there appeared to be a shift in the species composition of whitebait with much lower proportions of kōaro and banded kōkopu in 2015. Kōaro populations are highly susceptible to predation from trout and competition from smelt. These pressures have caused large declines in kōaro populations in the North Island (Rowe Citation1993; Rowe et al. Citation2002) that may be limiting the regional larval supply of kōaro. The reason(s) for the apparent reduction in the abundance of banded kōkopu whitebait in Bay of Plenty rivers is less clear. Adult banded kōkopu populations throughout New Zealand have been impacted by changes in land use from forest to pasture (Rowe et al. Citation1999). However, forested catchments in the Bay of Plenty (Rowe et al. Citation1999) and particularly on the nearby Coromandel Peninsula (West et al. Citation2005) still support very high densities of banded kōkopu. There is no evidence to suggest that reduced source populations might have resulted in a significant reduction in the abundance of banded kōkopu in the regional larval pool.
It is apparent that multiple factors interact to shape the species diversity in a whitebaiter's bucket. These include where they are fishing, when they are fishing, the type of net they are using (McDowall and Eldon Citation1980), how far they are fishing from the river mouth (McDowall and Eldon Citation1980), whether the river drains a forested catchment (Rowe et al. Citation1992), whether adult whitebait are present in the catchment (Baker and Hicks Citation2003) and whether it has rained heavily in the last few days (McDowall and Eldon Citation1980) together with a large amount of skill and luck. The whitebait fishery regulations must be sufficiently tailored and flexible to allow for this variability if they are to conserve the diversity of life-histories present in the catch and protect the whitebait fishery itself.
Supplementary Table 1
Download PDF (111.1 KB)Supplementary material
Download PDF (140.7 KB)Acknowledgements
Thank you to the many whitebaiters around the country that helped with fish collections; such a large survey would have been impossible without your time, effort, skills and perseverance. Thank you to the staff, particularly Jesse Burns, and students of the Marine Ecology Research Group for assistance with sampling and laboratory work. Thanks to Spencer Virgin for assistance with graphics. Outside-of-season sampling was completed with a Department of Conservation research permit (4436-FAU). This research was funded by Ministry of Business, Innovation and Employment grant C01X1002.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Allibone RM, Boubée JAT, West DW. 1999. The ones that got away: determining whitebait movements and rates of escape. Water Atmos. 7(1):11–13.
- Anderson MJ, Gorley RN, Clarke KR. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth: PRIMER-E Ltd.
- Augspurger JM. 2017. Early life history of a landlocked amphidromous fish: migration, critical traits and ontogeny [PhD thesis]. Dunedin: University of Otago.
- Baker CF, Hicks BJ. 2003. Attraction of migratory inanga (Galaxias maculatus) and koaro (Galaxias brevipinnis) juveniles to adult galaxiid odours. N Z J Mar Freshw Res. 37(2):291–299.
- Baker CF, Montgomery JC. 2001. Species-specific attraction of migratory banded kokopu juveniles to adult pheromones. J Fish Biol. 58(5):1221–1229.
- Baskaran R, Cullen R, Colombo S. 2009. Estimating values of environmental impacts of dairy farming in New Zealand. N Z J Agric Res. 52(4):377–389.
- Bonnett ML. 2000. Critical habitat features of giant kokopu, Galaxias argenteus (Gmelin 1789) [MSc thesis]. Christchurch: University of Canterbury.
- Boubée JAT, Dean TL, West DW, Barrier RFG. 1997. Avoidance of suspended sediment by the juvenile migratory stage of six New Zealand native fish species. N Z J Mar Freshw Res. 31(1):61–69.
- Charteris SC, Ritchie PA. 2002. Identification of galaxiid nests, emigrating larvae and whitebait, using mitochondrial DNA control region sequences. N Z J Mar Freshw Res. 36(4):789–795.
- Chiswell SM, Rickard GJ. 2011. Larval connectivity of harbours via ocean currents: a New Zealand study. Cont Shelf Res. 31(10):1057–1074.
- de Lange W, Bell RG, Gorman R, Reid S. 2003. Physical oceanography of New Zealand waters. In: Goff JR, Nichol S, Rouse HL, editors. The New Zealand coast: Te Tai o Aotearoa. Palmerston North: Dunmore Press; p. 59–78.
- Dijkstra LH, McDowall RM. 1997. Electrophoretic identification of whitebait species. Conserv Advis Sci Notes. 153:1–13.
- Egan EMC. 2017. Early life history of the amphidromous galaxiid īnanga: disentangling the consequences for their migratory dynamics, population structure and adult growth [PhD thesis]. Christchurch: University of Canterbury.
- Goodman JM, Dunn NR, Ravenscroft PJ, Allibone RM, Boubée JAT, David BO, Griffiths M, Ling N, Hitchmough RA, Rolfe JR. 2014. Conservation status of New Zealand freshwater fish, 2013. N Z Threat Classif Ser. 7:1–12.
- Hanchet SM. 1990. Effect of land use on the distribution and abundance of native fish in tributaries of the Waikato River in the Hakarimata Range, North Island, New Zealand. N Z J Mar Freshw Res. 24(2):159–171.
- Hickford MJH, Schiel DR. 2016. Otolith microchemistry of the amphidromous Galaxias maculatus shows recruitment to coastal rivers from unstructured larval pools. Mar Ecol Prog Ser. 548:197–207.
- Kelly GR. 1988. An inventory of whitebaiting rivers in the South Island. N Z Freshw Fish Rep. 101:1–65.
- May RM, Beddington JR, Clark CW, Holt SJ, Laws RM. 1979. Management of multispecies fisheries. Science. 205(4403):267–277.
- McClintock GJ. 2018. Early life history dynamics of the New Zealand whitebait species [MSc thesis]. Christchurch: University of Canterbury.
- McDowall RM. 1965. The composition of the New Zealand whitebait catch, 1964. N Z J Sci. 8(3):285–300.
- McDowall RM. 1984. The New Zealand whitebait book. Wellington: Reed.
- McDowall RM. 1990. New Zealand freshwater fishes: a natural history and guide. Auckland: Heinemann Reed.
- McDowall RM. 1999. Migration season of whitebait of giant kokopu, Galaxias argenteus. Conserv Advis Sci Notes. 263:1–9.
- McDowall RM. 2000. The Reed field guide to New Zealand freshwater fishes. Auckland: Reed.
- McDowall RM, Eldon GA. 1980. The ecology of whitebait migrations (Galaxiidae: Galaxias spp.). Fish Res Bull N Z Min Agr Fish. 20:1–172.
- McDowall RM, Jellyman DJ, Dijkstra LH. 1998. Arrival of an Australian anguillid eel in New Zealand: an example of transoceanic dispersal. Environ Biol Fishes. 51(1):1–6.
- McDowall RM, Kelly GR. 1999. Date and age at migration in juvenile giant kokopu, Galaxias argenteus (Gmelin) (Teleostei: Galaxiidae) and estimation of spawning season. N Z J Mar Freshw Res. 33(2):263–270.
- Rowe DK. 1993. Disappearance of koaro, Galaxias brevipinnis, from Lake Rotopounamu, New Zealand, following the introduction of smelt, Retropinna retropinna. Environ Biol Fishes. 36(4):329–336.
- Rowe DK, Chisnall BL, Dean TL, Richardson J. 1999. Effects of land use on native fish communities in east coast streams of the North Island of New Zealand. N Z J Mar Freshw Res. 33(1):141–151.
- Rowe DK, Hicks DM, Richardson J. 2000. Reduced abundance of banded kokopu (Galaxias fasciatus) and other native fish in turbid rivers of the North Island of New Zealand. N Z J Mar Freshw Res. 34(3):547–558.
- Rowe DK, Kelly G. 2009. Duration of the oceanic phase for inanga whitebait (Galaxiidae) is inversely related to growth rate at sea. In: Haro A, Smith KL, Rulifson RA, et al., editors. Challenges for diadromous fishes in a dynamic global environment. Halifax: American Fisheries Society; p. 343–354.
- Rowe DK, Konui G, Christie KD. 2002. Population structure, distribution, reproduction, diet, and relative abundance of koaro (Galaxias brevipinnis) in a New Zealand lake. J Roy Soc N Z. 32(2):275–291.
- Rowe DK, Saxton BA, Stancliff AG. 1992. Species composition of whitebait (Galaxiidae) fisheries in 12 Bay of Plenty rivers, New Zealand: evidence for river mouth selection by juvenile Galaxias brevipinnis (Günther). N Z J Mar Freshw Res. 26(2):219–228.
- Stancliff AG, Boubée JAT, Palmer D, Mitchell CP. 1988. The upstream migration of whitebait species in the lower Waikato River. N Z Freshw Fish Rep. 96:1–44.
- Stevens JCB, Hickford MJH, Schiel DR. 2016. Evidence of iteroparity in the widely distributed diadromous fish inanga Galaxias maculatus and potential implications for reproductive output. J Fish Biol. 89(4):1931–1946.
- Taylor MJ. 2002. The national inanga spawning database: trends and implications for spawning site management. Sci Cons. 188:1–37.
- Ueta Y, Tokai T, Segawa S. 1999. Relationship between year-class abundance of the oval squid Sepioteuthis lessoniana and environmental factors off Tokushima Prefecture, Japan. Fish Sci. 65(3):424–431.
- Urbina MA, Forster ME, Glover CN. 2011. Leap of faith: voluntary emersion behaviour and physiological adaptations to aerial exposure in a non-aestivating freshwater fish in response to aquatic hypoxia. Physiol Behav. 103(2):240–247.
- Waters JM, López JA, Wallis GP. 2000. Molecular phylogenetics and biogeography of galaxiid fishes (Osteichthyes: Galaxiidae): dispersal, vicariance, and the position of Lepidogalaxias salamandroides. Syst Biol. 49(4):777–795.
- West DW, Jowett IG, Richardson J. 2005. Growth, diet, movement, and abundance of adult banded kokopu (Galaxias fasciatus) in five Coromandel, New Zealand streams. N Z J Mar Freshw Res. 39(4):915–929.
