ABSTRACT
With ongoing climate change, aquaculture faces environmental challenges similar to those of natural ecosystems. These include increasing stress for calcifying species, e.g. macroalgae and shellfish. In this context, ocean acidification (OA) has the potential to affect important socioeconomic activities, including shellfish aquaculture, due to changes in the seawater carbonate system. However, coastal environments are characterised by strong diurnal pH fluctuations associated with the metabolic activity of macroalgae; that is, photosynthesis and respiration. This suggests that calcifying organisms that inhabit these ecosystems are adapted to this fluctuating pH environment. Macrophyte-dominated environments may have the potential to act as an OA buffering system in the form of a photosynthetic footprint, by reducing excess of CO2 and increasing the seawater pH and Ωarg. This can support calcification and other threatened physiological processes of calcifying organisms under a reduced pH environment. Because this footprint is supportive beyond the macroalgal canopy spatial area, this chemical refuge mechanism can be applied to support shellfish aquaculture, e.g. mussels. However, this approach should be tested in commercial shellfish farms to determine critical aspects of implementation. This includes critical factors such as target species and productivity rates. The degree of OA buffering capacity caused by the metabolic activity of macroalgae might depend on community structure and hydrodynamic conditions, creating site-specific responses. This concept might aid the development of future adaptive strategies, supporting marine ecological planning for the mussel aquaculture industry in Chile.
INTRODUCTION
The primary driver of climate change, atmospheric CO2 emissions, is producing dramatic variations in sea surface temperature and in the ocean carbonate system (Intergovernmental Panel on Climate Change Citation2013). The main effects of ongoing climate change include alterations in biodiversity, shifts in marine ecosystem regimes, increase in diseases, and a reduction of ecosystem services (Kroeker et al. Citation2013; Krumhansl et al. Citation2016; Méléder et al. Citation2010; Müller et al. Citation2009). Climate change may be particularly critical for food ecosystems sustained by coastal ocean processes with subsequent effects on social economy, human health and well-being, and human services to nature (Costa-Pierce Citation2016; Willett et al. Citation2019). Both wild fisheries and aquaculture, as part of the ocean food system, represent a major contribution to food security and adequate nutrition for the exponentially growing global human population (Food and Agriculture Organization [FAO 2016]; Harvey et al. Citation2017).
After China, Chile is the second largest global producer of mussels, producing 302,000 tonnes in 2018 (SeafoodSource Citation2019). Production increased by 20% during the 2006–2016 period, contributing approximately 1.2 million tonnes to global production (Servicio Nacional de Pesca y Acuicultura [SERNAPESCA] Citation2018). In Chile, monocultures of fish and Molluscs have become the main resources for aquaculture, with a limited production of seaweeds (SERNAPESCA Citation2018). Therefore, the greatest challenges for Chilean aquaculture are product diversification and improvement of aquaculture techniques (Buschmann et al. Citation1996a, Citation2008a, Citation2013). Facing the negative effects of environmental drivers associated with human activities, e.g. climate change on marine ecosystems, aquaculture diversification may become critical for sustaining production (Harvey et al. Citation2017; Troell Citation2009).
Earth’s oceans absorb more than one third of global anthropogenic CO2 emissions, altering seawater carbonate chemistry and leading to a process known as ocean acidification (OA; Resplandy et al. Citation2018). OA involves a decrease in pH, a reduction in the concentration of carbonate ions (CO32−), and a decline of the saturation state (Ω) of calcium carbonate (CaCO3) mineral forms, i.e. calcite and aragonite (Caldeira & Wickett Citation2003). These chemical changes in seawater may effect calcifying marine organisms, in particular, corals, coralline macroalgae and shell-forming molluscs (Harley et al. Citation2012; Koch et al. Citation2013). Impacts of OA are particularly strong during the early life stages of marine calcifying species. This has been observed with spores of macroalgae and free-living planktonic larvae of benthic invertebrates, altering biodiversity and productivity of coastal ecosystems (Chen et al. Citation2019; Frieder et al. Citation2014; Leal et al. Citation2018; Przeslawski et al. Citation2015).
Many benthic invertebrates, including calcifying organisms, play an important ecological role in marine ecosystems but are also directly used by humans as fishery resources (Onitsuka et al. Citation2018). World shellfish production – for example, oysters, cockles, clams, scallops, abalone and mytilids – has reached approximately 16 million tonnes (FAO 2016). Therefore, the expected OA impacts on shell-forming organisms may become a global social–ecological problem (Forsyth et al. 2008). This is particularly relevant for Chilean aquaculture where shellfish molluscs represent 28% of national production (SERNAPESCA Citation2018). Significantly, Chile’s main cultivated species, the blue mussel (Mytilus chilensis), accounts for approximately 15% of global cultivated production (FAO 2016). Moreover, M. chilensis and related species (Choromytilus chorus, Aulacomya ater) play important ecological roles in marine ecosystems, providing biogenic habitats for benthic ecosystems and promoting the recycling of organic matter (Brattström & Johanssen Citation1983; Häussermann & Försterra Citation2009). Therefore, strategies to mitigate the negative impacts of OA on ecologically and economically important shell-forming organisms are urgently needed.
The reduction of CO32− concentration under OA conditions () increases the vulnerability of early life stages of bivalves. OA affects metabolic energy budgets, impairs neurological function, alters behaviour, and causes shell dissolution, all leading to a reduction in growth and survivorship (Frieder et al. Citation2014; Green et al. Citation2013; Kurihara Citation2008; Waldbusser et al. Citation2015). In bivalves, calcification is the biochemical production of calcified shells to support and protect their soft bodies (Gazeau et al. Citation2013; Marin et al. Citation2000). Adult bivalve shells are typically formed by aragonite and/or calcite, which are sensitive to seawater pH (Doney et al. Citation2009; Gazeau et al. Citation2013; Roleda et al. Citation2012). In acidified waters, the Ωcal and Ωarg are reduced to below 1.0 because CO32− concentration also declines (Caldeira & Wickett Citation2003; Doney et al. Citation2009). This condition makes the production and maintenance of shell or skeletal structures more difficult because shell production is outcompeted by shell dissolution (). As a result, more energy has to be allocated to support calcification, leading to a potential reduction in other physiological processes such as growth and reproduction (Gazeau et al. Citation2013; Hendriks et al. Citation2015; Roleda et al. Citation2012).
Fig. 1. Conceptual diagram of possible interactions between macroalgae and mussels in co-culture under OA conditions. Seawater pH and aragonite saturation state (Ωarg) are reduced to unfavourable conditions for calcification. During daytime, macroalgal photosynthesis increases pH in surrounding seawater, counteracting OA effects on calcification. However, during nighttime, macroalgae and mussels release CO2 during respiration, contributing to OA conditions. This last condition can be different if appropriate culture proportions are previously determined in, for example, laboratory experiments. Moreover, photosynthetic O2 released during daytime and macroalgal detritus can benefit mussels metabolism, and excretion of NH4+, urea and CO2 can support macroalgal growth and photosynthesis. Mussels can also help control phytoplankton blooms by filtration. Note: arrow size indicates direction in which the reaction is favoured (nondashed, negative; dashed; positive).
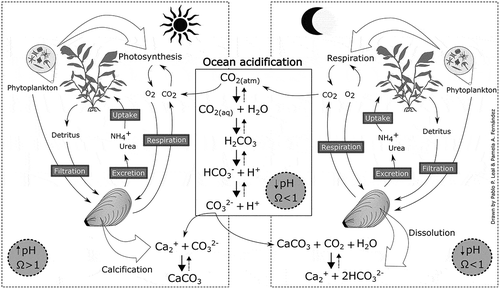
Primary producers like macroalgae have a large capacity to fix CO2 via photosynthesis and take up dissolved inorganic nutrients such as phosphorus and nitrogen (Chung et al. Citation2013; Harrison & Hurd Citation2001; Hurd et al. Citation2014). Their growth is also supported by other essential minerals and trace metals (Mišurcová Citation2012). Therefore, it is not surprising that, due to their large capacity to take up dissolved inorganic nutrients, they have been used in integrated multitrophic aquaculture (IMTA). IMTA combines the cultivation of finfish, filter-feeding shellfish and macroalgae, with the main objective of mitigating the metabolic waste generated by the main cultivated species (Roleda & Hurd Citation2019; Troell et al. Citation2009; Turan & Neori Citation2010). This concept has been extensively studied and developed in several countries, including the United States, China, Canada, and Chile (Abreu et al. Citation2009; Buschmann et al. Citation2008b; Chopin et al. Citation2008, Citation2012; Fang et al. Citation2016; Mao et al. Citation2009; Troell et al. Citation2009, Citation2014). However, due to the associated cost of finfish cultivation and/or environmental uncertainty of breeding finfish offshore, IMTA has moved towards a co-culture concept, combining only shellfish and seaweeds (Ladner et al. Citation2018). It has become apparent that the co-culture of these species has important benefits in coastal ecosystems. They absorb high amounts of carbon (C) via photosynthesis and by filter feeding on particulate organic matter (Han et al. Citation2017; Tang et al. Citation2011). Furthermore, the co-culture of shellfish and macroalgae could provide a natural mechanism to buffer the negative impacts of OA on marine ecosystems (, ). This can enhance the early development and growth of wild and farmed shellfish and might also increase the potential for large-scale macroalgal cultivation. Today, this represents only 25% of global aquaculture; however, it is concentrated mainly in China (Chung et al. Citation2013; Fang et al. Citation2016; Xiao et al. Citation2017).
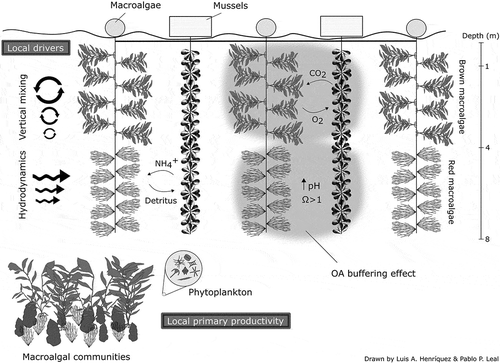
Fig. 2. Schematic representation of co-culture of mussels and macroalgae and its possible relationship with local environment. In this co-culture system, mussels and macroalgae are alternately placed to take advantage of their respective metabolic products. For example, excretion of NH4+ and urea by mussels can be used by macroalgae and macroalgal detritus may feed mussels. OA buffering refuge created by macroalgal photosynthesis may benefit calcification of proximate cultured mussels. However, these interactions between co-cultured organisms can be affected by inherent species’ production rates and local drivers such hydrodynamics and vertical mixing.
Macrophytes as OA refuges for calcifying marine organisms
The concept of chemical refuge has been raised in response to major concerns about the negative effects of OA on calcifying invertebrates (Buapet et al. Citation2013; Greiner et al. Citation2018; Groner et al. Citation2018; Hendriks 2014; Krause-Jensen et al. Citation2016; Unsworth et al. Citation2012). In coastal ecosystems, macroalgal beds are characterised by intense metabolic activity capable of modifying their surrounding environment (Cornwall et al. Citation2013, Citation2014; Dayton Citation1985; Hofmann et al. Citation2011; Hurd Citation2015). For example, during daytime, macroalgae remove CO2 by photosynthesis from the proximate seawater, increasing the pH of surrounding seawater up to 8.8. This can produce favourable conditions for shellfish calcification (i.e. Ω > 1; ; Caldeira & Wickett Citation2003, Doney et al. Citation2009). During the night, respiration releases CO2 to the external environment (), which in turn can reduce surrounding seawater pH to 7.8 (Cornwall et al. Citation2013; Hofmann et al. Citation2011). Other studies have indicated that reduced pH during the night within macrophyte assemblages can have negative effects on bivalve calcification (Saderne et al. Citation2015). Hendriks et al. (Citation2015) measured a beneficial interaction between these organisms in both natural and controlled environments. For example, under experimental conditions, semidiurnal variations of pH ameliorate the negative effect of static reduced pH on the development of early life stages of mytilid and coral species. This shows that even a short exposure to higher pH might help calcifying organisms tolerate OA conditions (Enochs et al. Citation2018; Frieder et al. Citation2014). Consequently, it has been suggested that calcifying marine organisms living in fluctuating coastal environments have evolved a wide range of mechanisms to cope better with changing pH. These include pH upregulation in extracellular fluids and synchronisation of the calcification window for the most productive hours of the day, when CO2 is removed via photosynthesis (Hendriks et al. Citation2015; McCulloch et al. Citation2012).
However, field observations have shown contrasting effects of natural assemblages of macrophytes on calcification rates of calcifying organisms. Meadows of the seagrass Zostera marina Linnaeus can enhance calcification of the calcareous red alga Hydrolithon sp. (Semesi et al. Citation2009). In contrast, beds of the brown macroalga Padina pavonica (Linnaeus) Thivy and meadows of Z. marina were unable to prevent adverse effects of acidification on epiphytic foraminifera and mussels, respectively (Martin et al. Citation2008; Pettit et al. Citation2015; Saderne et al. Citation2015). To date, only one study has investigated the effects of macrophyte presence close to shellfish cultures and how their OA buffering capacity can support calcification (Greiner et al. Citation2018). This study indicated that the presence of macrophytes does not increase pH or Ωarg, suggesting that this might not be the best strategy to improve carbonate seawater chemistry near clam beds. These contradictory examples on the possible OA chemical refuge for calcifying organisms suggest that further research is needed to determine whether photosynthetic organisms can consistently create a favourable environment for calcification.
The degree of OA buffering capacity might depend on the structure of macrophyte communities and hydrodynamic conditions. This would mean site-specific responses (Comeau & Cornwall Citation2017; Greiner et al. Citation2018). In a habitat formed by high biomass such as macroalgae or seagrasses, seawater pH can reach optimal values for calcification of associated organisms (Cornwall et al. Citation2013; Pettit et al. Citation2015; Saderne et al. Citation2015). Reciprocally, in natural environments, shellfish excrete NH4+ and urea, which favour growth of macroalgae (Chung et al. Citation2013; Duarte et al. Citation2017). Moreover, shellfish respiration releasing CO2 may benefit macroalgal photosynthesis but also reduce seawater pH, which can be detrimental for calcification. Therefore, because metabolic rates are species specific, it is necessary to determine the optimal ratio between the two cultivated species to obtain an efficient utilisation of CO2 (uptake) and the extent of the buffering OA effect. Although the introduction of macroalgae near shellfish culture might be a good strategy to mitigate negative impacts of OA on economically important bivalve species such as mussels (Billé et al. Citation2013; Greiner et al. Citation2018; Hendriks et al. Citation2015), this needs to be investigated under laboratory and large-scale field conditions.
Strategies to utilise inorganic carbon (Ci) such as CO2 and HCO3− from seawater to support photosynthesis vary between species. These strategies include carbon concentrating mechanisms (CCMs; Raven et al. Citation2012). Macroalgae that depend on purely diffusive entry of CO2 through the plasma membrane (also known as non-CCM species) are thought to benefit under an OA scenario, by increasing their photosynthesis and growth (Cornwall et al. Citation2017). Macroalgae with a CCM (CCM species) have developed different mechanisms to use HCO3− via external dehydration mediated by the enzyme carbonic anhydrase and/or active uptake through an ATPase pump or anion port. This is predicted to show a neutral or positive response depending on enzyme affinity and mechanisms to use HCO3− (Fernández et al. Citation2014; Hepburn et al. Citation2011). Similar to the non-CCM species, CCM species with low Ci affinity but high capacity to downregulate their CCMs may also benefit under elevated CO2. However, CCM species with high Ci affinity but lower capacity to regulate their CCMs may not respond to subsequent changes in CO2 (Cornwall et al. Citation2017; Hepburn et al. Citation2011). Experimental surveys have determined that > 50% of red macroalgae are non-CCM and that > 80% of brown and green macroalgae are CCM (Cornwall et al. Citation2015; Hepburn et al. Citation2011). Therefore, we could expect that macroalgae that benefit from elevated CO2 via increased Ci availability to support photosynthesis would have a higher capacity to increase seawater pH and modify seawater carbonate chemistry in their surrounding environment. This may be beneficial for cultivating calcifying organisms under an OA scenario.
The Chilean coast is characterised by wide fluctuations in pH (pulses of high CO2 and low pH) associated with upwelling events (Torres et al. Citation2011) and influenced by input of freshwater due to river discharges, generating estuarine systems (Häussermann & Försterra Citation2009; Pantoja et al. Citation2011; Silva & Vargas Citation2014; Vargas et al. Citation2016). Estuarine waters are characterised by low alkalinity, pH and carbonate ions (Duarte et al. Citation2013; Waldbusser & Salisbury Citation2014). These conditions might result in low Ωarg, affecting calcifying organisms that inhabit these coastal systems. In Chile, aquaculture of M. chilensis occurs in both coastal and estuarine systems and, hence, is naturally exposed to a fluctuating pH environment, ranging from 7.40 in estuarine areas to 8.10 in areas not influenced by freshwater (Duarte et al. Citation2015). This is important because in environments with naturally reduced pH – for example, estuarine systems – the negative effects of OA may be exacerbated (Cai et al. Citation2011). In addition, the negative effects of OA on physiological responses – that is, growth – of M. chilensis suggest that OA might have important consequences on mussel aquaculture in Chile (Duarte et al. Citation2014; Navarro et al. Citation2013, Citation2016). Nevertheless, intraspecific variability may also be found (Duarte et al. Citation2015). Therefore, in this context, in ecosystems such as Chilean estuaries, the introduction of macroalgae near or within shellfish farms may ameliorate the negative impact of OA ().
The need for implementing shellfish–macroalgae co-culturing: Towards OA–IMTA aquaculture
The most immediate challenge for the implementation of long-term sustainable integrated aquaculture is preventing environmental impacts. This is possible through efficient utilisation of particulate and dissolved metabolic waste such as N and P generated by the cultivated species (Fang et al. Citation2016; Granada et al. Citation2016; Troell et al. Citation2009). However, the need for carbon fixation has become increasingly important given the increases in CO2 concentration and because of the capacity of coastal ecosystems to sequester carbon (Billé et al. Citation2013; Laffoley & Grimsditch Citation2009). Accordingly, management of coastal carbon by aquaculture could ameliorate the global climate crisis and address adverse scenarios for shellfish production. In this sense, defining an optimal culture design is required to ensure efficient recycling of byproducts in multitrophic systems; that is, IMTA practice. In addition, it will act as a CO2 sink and maximise the seaweed OA buffering effect (). Experience gained from experiments, such as small-scale IMTA operations under controlled conditions, will be instrumental for realising OA strategy mitigation for farmed shellfish. In this context, site- and species-specific responses in different physical conditions are equally relevant to improving biomass production in co-culture farms. These aims may be co-ordinated with successful commercial IMTA systems mainly from the coasts of Canada and China, where co-cultivation of salmon, blue mussel, abalone, scallops and macroalgae have both ecological and economic benefits (Granada et al. Citation2016; Ridler et al. Citation2007; Troell Citation2009).
Optimal co-culturing proportions between macroalgae and bivalves as well as knowledge of the best harvest period for each cultivated species (seasonal variability) need to be considered to realise synergistic growth effects (Ladner et al. Citation2018). Han et al. (Citation2017) suggested that a co-culturing ratio c. 4:1 between Crassostrea angulata and Gracilaria lemaneiformis (Bory) Greville results in efficient utilisation of dissolved inorganic carbon by G. lemaneiformis. Han et al. (Citation2017) showed that a significant increase of pH favoured calcification of C. angulata and enhanced the local CO2 sink. This ratio was comparable to those reported by Mao et al. (Citation2009) and Ajjabi et al. (Citation2018), who found that a bivalve:macroalgae culturing ratio of c. 3:1 was advisable for more efficient NH+4 and P uptake for seaweed growth. These results suggest that at large scales, a substantial OA buffering environment is possible, while maintaining optimal nutrient removal and growth benefits.
Another positive bivalve–macroalgal trophic relationship is algal detritus as a source of C for bivalves (Peterson et al. Citation1984; Wiedemeyer & Schwamborn Citation1996). In an IMTA system, Xu et al. (Citation2016) established, via the carbon isotope method, that 14%–42% of C in the scallop Chlamys farreri was obtained from the kelp Saccharina japonica (Areschoug) C.E.Lane, C.Mayes, Druehl & G.W.Saunders. This enhancement of the food supply may be beneficial for growth under a reduced pH environment by allowing for the allocation of more energy resources for calcification (Hendriks et al. Citation2015). These relationships are tied to complex interactions within the environment (Chopin et al. Citation2008; Fang et al. Citation2016; Newell Citation2007) and to the physiological responses of each species. These can be modulated by factors such as light, stocking density, water depth, water flow and renewal rate (Buschmann et al. Citation1996b; Huo et al. Citation2012; Mao et al. Citation2009). This should be investigated in the context of the OA buffering effect by macroalgae.
Prevailing hydrodynamic regimes and vertical mixing may be critical for the design of an effective cultivation layout. Studies on the seagrass Posidonia oceanica (Linnaeus) Delile indicated that the footprint of the OA buffering effect can be detected approximately 10 m from the meadow boundary. Furthermore, mussel farms tend to reduce current speeds, which affects water residence times (Hendriks et al. Citation2014; Keeley et al. Citation2009). This may accentuate the effects of the macroalgal OA buffering effect within the limit of the farm (). However, reduced current speed might affect macroalgal nutrient uptake via increasing the diffusion boundary layer (Hurd Citation2000). In turn, bivalves and associated fauna (i.e. from fouling) release NH4+, faeces and pseudofaeces directly into the water column (Coen et al. Citation2007; Dumbauld et al. Citation2009; Ferriss et al. Citation2016; Keeley et al. Citation2009), increasing nutrient concentration in the seawater. This counteracts the effects of slow nutrient transport across a thicker diffusion boundary layer (Hurd Citation2000). However, large-scale co-culturing of shellfish–macroalgae in a relatively static system may promote accumulation of faeces and pseudofaeces on macroalgal thalli (Newell Citation2007), affecting physiological processes such as photosynthesis. For example, Jiang et al. (Citation2015) showed that excess suspended particulate matter (e.g. < 63 μm grain size) reduced the viability of microscopic stages of foliose and crustose coralline algae by reducing their photosynthetic efficiency (Airoldi Citation2003; Harrington et al. Citation2005). In addition, biodeposition can enhance ephemeral macroalgae with stronger effects on sheltered coastal areas (Kotta et al. Citation2009; Madsen et al. Citation2001). These studies highlight the importance of investigating hydrodynamic regimes when considering the location for a co-culture of shellfish and macroalgae.
In channels and fjords from southern Chile (41° to 56°S), local current speed and water circulation are influenced by tidal oscillations, freshwater inputs, and wind variation (Castillo & Valenzuela Citation2008). Tidal currents tend to vary from relatively weaker in deep fjord systems (c. 0.1 m−1 s−1) to stronger (c. 0.7 m−1 s−1) in shallower channels (Instituto de Fomento Pesquero Citation2019; Valle-Levinson et al. Citation2007). This is relevant for developing an efficient mechanism to recapture inorganic and organic metabolic waste generated by the cultivated carnivore species and to identify the dispersal and retention zones across the region. In Chile, as in other countries, mussel aquaculture encompasses two production cycles: a seeding period (from early to late spring) and a growth period (from early autumn to late spring). Accordingly, the mussel growth period seems to be more relevant for introduction of macroalgae and the attenuation of adverse impacts from particulate waste on macroalgal thalli. Southerly winds dominate from early autumn to early spring and tend to be stronger than in late spring and summer, promoting surface turbulence and vertical mixing (Valle-Levinson et al. Citation2007). This agrees with the season (autumn–spring) for optimum seeding and growth for farming Macrocystis pyrifera (Linnaeus) C.Agardh (Camus et al. Citation2019). In addition, the limited light environment during the low productivity period (autumn–winter) restricts the growth of nuisance epiphytic algae in pilot crops of commercial macroalgae (Avila et al. Citation1999; Lüning & Pang Citation2003; Romo et al. Citation2001; Westermeier et al. Citation1993). However, as mentioned above, temporal constraints should be considered because the optimum growth season can vary among cultivated species.
Macroalgal growth might be limited by the vertical attenuation of photosynthetically active radiation and shading by adjacent lines and culture sleeves. Depth is a limiting factor for the cultivation of macroalgae and restricts productivity to the first few metres of the water column (Beer et al. Citation2016). Therefore, we propose that macroalgal cultivation on vertical lines be tested to determine both OA buffering effects and resulting productivity rates (). This is critical for important commercial species such as Agarophyton chilensis (C.J.Bird, McLachlan & E.C.Oliveira) Gurgel, J.N.Norris & Fredericq (formerly Gracilaria chilensis; Gurgel et al. Citation2018), in which productivity may vary from 84 to 132 t ha−1 year−1 by increasing culturing depth from 0.75 to 1.5 m, respectively (Buschmann et al. Citation1997; see Espi et al. 2019 for review of seaweed aquaculture in Latin America). For M. pyrifera, effective suspended growth has been determined to occur at c. 3-m depth (Varela et al. Citation2018). However, the presence of accessory pigments phycoerythrin and phycocyanin in red macroalgae (Rhodophyta) becomes relevant for cultivation under light limitation and can help determine optimal cultivation depth (). For instance, during early growth of Gigartina skottsbergii Setchell & N.L.Gardner optimal depths are between 4 and 6 m (Romo et al. Citation2006); Sarcothalia crispata (Bory) Leister had higher growth rates at 12–13 m (Westermeier et al. Citation2012); and Chondracanthus chamisoii (C.Agardh) Kützing can be found growing naturally at depths up to 15 m on the central coast of Chile (Hoffmann & Santelices Citation1997). Overall, these outcomes suggest that culturing red macroalgae below 4-m depth along with filter-feeder sleeves may provide a successful strategy. This can maximise both the macroalgal OA buffering effect in greater portions of the water column and the adaptive mechanisms to grow under attenuated light (). In addition, red algae are characterised by using both CO2 and HCO3− as Ci forms to support photosynthesis (Maberly et al. Citation1992); hence, depending on their CCMs, some red algae might have increased photosynthetic rates under elevated CO2. Therefore, preliminary studies investigating the CCMs of the target species will be necessary to select the best candidate with respect to capacity to improve seawater carbonate chemistry.
Finally, another issue to be examined is the potential relationship of macroalgae as substrata for shellfish during recruitment. The use of a biogenic habitat, provided by seagrasses, drifting algae and algal rafts, for recruitment and dispersion has been documented for mussel larvae and spat in New Zealand (Alfaro et al. Citation2004; Jeffs et al. Citation2018). However, this may also increase the effects of epibionts and opportunistic macroalgae such as Desmarestia sp. (Phaeophyceae), which can affect mussel cultures during spring in southern Chile (local mussel growers, personal communication, November 2018).
CONCLUSIONS
OA is occurring faster than expected, and mitigation strategies are urgently needed to reduce anthropogenic CO2 emissions and their associated negative effects on marine ecosystems, coastal communities (e.g. calcifying organisms), and economic sectors (e.g. fisheries and aquaculture). Coastal environments are characterised by strong diurnal pH fluctuations associated with metabolic activity of macroalgae. Marine organisms inhabiting coastal zones have developed a wide range of mechanisms to cope with this changing pH environment. In this context, mimicking the natural OA buffering effect created via the photosynthetic footprint (i.e. reduction of CO2 and increase in seawater pH and Ωarg), which favours calcification, may be a strategy to mitigate the negative effects of OA on shell-forming molluscs. Beneficial interactions between macroalgae and calcifying organisms have been observed in natural environments and in co-culturing conditions; for example, efficiency of macroalgae to remove excess inorganic and organic metabolic waste generated by the cultured animals. However, these positive synergistic effects have not been investigated under an OA scenario. In this context, important factors related to hydrodynamic regimes and physiological responses of cultivated species (e.g. nutrient uptake, excretion rates, buffering effects and CO2 sink capacity) must be tested when considering a co-culture design. Thus, any potential benefit between these organisms may be both site specific and species specific and affect final biomass production. In addition, an ecosystem-based marine planning approach is needed in Chile to optimise different local possibilities. Policy is needed to maximise the benefits of multitrophic aquaculture for an industry that may need to adapt rapidly under climate change.
Acknowledgements
Many thanks to Jeremy Anbleyth-Evans for proofreading the last version of the article and correcting grammatical errors.
Additional information
Funding
References
- Abreu M.H., Varela D.A., Henríquez L.A., Villarroel A., Yarish C., Sousa-Pinto I. & Buschmann A.H. 2009. Traditional vs. integrated multi-trophic aquaculture of Gracilaria chilensis C. J. Bird, J. McLachlan & E. C. Oliveira: productivity and physiological performance. Aquaculture 293: 211–220. DOI: 10.1016/j.aquaculture.2009.03.043.
- Airoldi L. 2003. The effects of sedimentation on rocky coast assemblages. Oceanography and Marine Biology: An Annual Review 41: 161–236.
- Ajjabi L.C., Abaab M. & Segni R. 2018. The red macroalga Gracilaria verrucosa in co-culture with the Mediterranean mussels Mytilus galloprovincialis: productivity and nutrient removal performance. Aquaculture International 26: 253–266. DOI: 10.1007/s10499-017-0206-2.
- Alemañ A.E., Robledo D. & Hayashi L. 2019. Development of seaweed cultivation in Latin America: current trends and future prospects. Phycologia. 58: 462–471. DOI: 10.1080/00318884.2019.1640996.
- Alfaro A.C., Jeffs A.G. & Creese R.G. 2004. Bottom-drifting algal/mussel spat associations along a sandy coastal region in northern New Zealand. Aquaculture 241: 269–290. DOI: 10.1007/s10499-017-0206-2.
- Avila M., Ask E., Rudolph B., Nuñez M. & Norambuena R. 1999. Economic feasibility of Sarcothalia (Gigartinales, Rhodophyta) cultivation. Hydrobiologia 398/399: 435–442. DOI: 10.1023/A:1017077827860.
- Beer S., Björk M. & Beardall J. 2016. Photosynthesis in the marine environment. John Wiley & Sons, Iowa, USA. 244 pp.
- Billé R., Kelly R., Biastoch A., Harrould-Kolieb E., Herr D., Joos F., Kroeker K., Laffoley D., Oschlies A. & Gattuso J.-P. 2013. Taking action against ocean acidification: a review of management and policy options. Environmental Management 52: 761–779. DOI: 10.1007/s00267-013-0132-7.
- Brattström H. & Johanssen A. 1983. Ecological and regional zoogeography of the marine benthic fauna of Chile. Sarsia 68: 289–339. DOI: 10.1007/s00267-013-0132-7.
- Buapet P., Gullström M. & Björk M. 2013. Photosynthetic activity of seagrasses and macroalgae in temperate shallow waters can alter seawater pH and total inorganic carbon content at the scale of a coastal embayment. Marine and Freshwater Research 64: 1040–1048. DOI: 10.1071/MF12124.
- Buschmann A.H., Hernández-González M.C., Aranda C.P., Chopin T., Neori A., Halling C. & Troell M. 2008a. Mariculture waste management. In: Applications in ecological engineering (Ed. by S.E. Jorgensen & B.D. Fath), pp. 2211–2217. Elsevier, Oxford, UK.
- Buschmann A.H., López D.A. & Medina A. 1996a. A review of the environmental effects and alternative production strategies of marine aquaculture in Chile. Aquaculture Engineering 15: 397–421. DOI: 10.1016/S0144-8609(96)01006-0.
- Buschmann A.H., Retamales C.A. & Figueroa C. 1997. Ceramialean epiphytismin an intertidal Gracilaria chilensis (Rhodophyta) bed in southern Chile. Journal of Applied Phycology 9: 129–135. DOI: 10.1023/A:1007971615801.
- Buschmann A.H., Stead R.A., Hernández-González M.C., Pereda S.V., Paredes J.E. & Maldonado M.A. 2013. Critical analysis on the use of macroalgae as a base for sustainable aquaculture. Revista Chilena De Historia Natural 86: 251–264. DOI: 10.4067/S0716-078X2013000300003.
- Buschmann A.H., Troell M., Kautsky N. & Kautsky L. 1996b. Integrated tank cultivation of salmonids and Gracilaria chilensis (Gracilariales, Rhodophyta). Hydrobiologia 326: 75–82. DOI: 10.1007/BF00047789.
- Buschmann A.H., Varela D.A., Hernández-González M.C. & Huovinen P. 2008b. Opportunities and challenges for the development of an integrated seaweed-based aquaculture activity in Chile: determining the physiological capabilities of Macrocystis and Gracilaria as biofilters. Journal of Applied Phycology 20: 571–577. DOI: 10.1007/978-1-4020-9619-8_17.
- Cai W.J., Hu X., Huang W.J., Murrell M.C., Lehrter J.C., Lohrenz S.E., Chou W.C., Zhai W., Hollibaugh J.T., Wang Y. et al. 2011. Acidification of subsurface coastal waters enhanced by eutrophication. Nature Geoscience 4: 766–770. DOI: 10.1038/ngeo1297.
- Caldeira K. & Wickett M.E. 2003. Anthropogenic carbon and ocean pH. Nature 425: 365. DOI: 10.1038/425365a.
- Camus C., Infante J. & Buschmann A.H. 2019. Revisiting the economic profitability of giant kelp Macrocystis pyrifera (Ochrophyta) cultivation in Chile. Aquaculture 502: 80–86. DOI: 10.1016/j.aquaculture.2018.12.030.
- Castillo M. & Valenzuela C. 2008. Circulation regime in the austral Chilean channels and fjords. In: Progress in the oceanographic knowledge of Chilean interior waters, from Puerto Montt to Cape Horn (Ed. by N. Silva & S. Palma), pp. 59–62. Comité Oceanográfico Nacional, Pontificia Univ. Católica de Valparaiso, Chile.
- Chen B., Zou D., Ma Z., Yu P. & Wu M. 2019. Effects of light intensity on the photosynthetic responses of Sargassum fusiforme seedlings to future CO2 rising. Aquaculture Research 50: 116–125. DOI: 10.1111/are.2019.50.issue-1.
- Chopin T., Cooper J.A., Reid G., Cross S. & Moore C. 2012. Open-water integrated multi-trophic aquaculture: environmental biomitigation and economic diversification of feed aquaculture by extractive aquaculture. Reviews in Aquaculture 4: 209–220. DOI: 10.1111/j.1753-5131.2012.01074.x.
- Chopin T., Robinson S.M.C., Troell M., Neori A., Buschmann A.H. & Fang J. 2008. Multitrophic integration for sustainable marine aquaculture. In: Ecological engineering, vol. 3 (Ed. by S.E. Jørgensen & B.D. Fath), pp. 2463–2475. Elsevier, Oxford, UK.
- Chung I.K., Oak J.H., Lee J.A., Shin J.A., Kim J.G. & Park K.S. 2013. Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean project overview. ICES Journal of Marine Science 70: 1038–1044. DOI: 10.1093/icesjms/fss206.
- Coen L.D., Brumbaugh R.D., Bushek D., Grizzle R., Luckenbach M.W., Posey M.H., Powers S.P. & Tolley S.G. 2007. Ecosystem services related to oyster restoration. Marine Ecology Progress Series 341: 303–307. DOI: 10.3354/meps341303.
- Comeau S. & Cornwall C.E. 2017. Contrasting effects of ocean acidification on coral reef “animal forests” versus seaweed “kelp forests.”. In: Marine animal forests, the ecology of benthic biodiversity hotspots (Ed. by S. Rossi, L. Bramanti, A. Gori & C. Orejas), pp. 1–43. Springer International Publishing, Cham, Switzerland.
- Cornwall C.E., Boyd P.W., McGraw C.M., Hepburn C.D., Pilditch C.A., Morris J.N., Smith A.M. & Hurd C.L. 2014. Diffusion boundary layers ameliorate the negative effects of ocean acidification on the temperate coralline macroalga Arthrocardia corymbosa. PLoS ONE 9: e97235. DOI: 10.1371/journal.pone.0097235.
- Cornwall C.E., Hepburn C.D., McGraw C.M., Currie K.I., Pilditch C.A., Hunter K.A., Boyd P.W. & Hurd C.L. 2013. Diurnal fluctuations in seawater pH influence the response of a calcifying macroalga to ocean acidification. Proceedings of the Royal Society B 280: 20132201. DOI: 10.1098/rspb.2013.2201.
- Cornwall C.E., Revill A.T., Hall-Spencer J.M., Milazzo M., Raven J.A. & Hurd C.L. 2017. Inorganic carbon physiology underpins macroalgal responses to elevated CO2. Scientific Reports 7: 46297. DOI: 10.1038/srep46297.
- Cornwall C.E., Revill A.T. & Hurd C.L. 2015. High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynthesis Research 124: 181–190. DOI: 10.1007/s11120-015-0114-0.
- Costa-Pierce B.A. 2016. Ocean foods ecosystems for planetary survival in the anthropocene. World Nutrition Forum 2016: 301–320.
- Dayton P.K. 1985. Ecology of kelp communities. Annual Review of Ecology, Evolution, and Systematics 16: 215–245. DOI: 10.1146/annurev.es.16.110185.001243.
- Doney S.C., Fabry V.J., Feely R.A. & Kleypas J.A. 2009. Ocean acidification: the other CO2 problem. Annual Review of Marine Science 1: 169–192. DOI: 10.1146/annurev.marine.010908.163834.
- Duarte C., Navarro J.M., Acuña K., Torres R., Manríquez P.H., Lardies M.A., Vargas C.A., Lagos N.A. & Aguilera V. 2014. Combined effects of temperature and ocean acidification on the juvenile individuals of the mussel Mytilus chilensis. Journal of Sea Research 85: 308–314. DOI: 10.1016/j.seares.2013.06.002.
- Duarte C., Navarro J.M., Acuña K., Torres R., Manríquez P.H., Lardies M.A., Vargas C.A., Lagos N.A. & Aguilera V. 2015. Intraspecific variability in the response of the edible mussel Mytilus chilensis (Hupe) to ocean acidification. Estuaries and Coasts 38: 590–598. DOI: 10.1007/s12237-014-9845-y.
- Duarte C.M., Hendriks I.E. Moore T.S., Olsen Y.S., Steckbauer A., Ramajo L., Carstensen J., Trotter J.A. & McCulloch M. 2013. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries and Coasts 36: 221–236. DOI: 10.1007/s12237-013-9594-3.
- Duarte C.M., Wu J., Xiao X., Bruhn A. & Krause-Jensen D. 2017. Can seaweed farming play a role in climate change mitigation and adaptation? Frontiers in Marine Science 4: 100. DOI: 10.3389/fmars.2017.00100.
- Dumbauld B.R., Ruesink J.L. & Rumrill S.S. 2009. The ecological role of bivalve shellfish aquaculture in the estuarine environment: a review with application to oyster and clam culture in west coast (USA) estuaries. Aquaculture 290: 196–223. DOI: 10.1016/j.aquaculture.2009.02.033.
- Enochs I.C., Manzello D.P., Jones P.J., Aguilar C., Cohen K., Valentino L., Schopmeyer S., Lolodziej G., Jankulak M. & Lirman D. 2018. The influence of diel carbonate chemistry fluctuations on the calcification rate of Acropora cervicornis under present day and future acidification conditions. Journal of Experimental Marine Biology and Ecology 506: 135–143. DOI: 10.1016/j.jembe.2018.06.007.
- Fang J., Zhang J., Xiao T., Huang D. & Liu S. 2016. Integrated multi-trophic aquaculture (IMTA) in Sanggou Bay, China. Aquaculture Environment Interactions 8: 201–205. DOI: 10.3354/aei00179.
- Fernández P.A., Hurd C.L. & Roleda M.Y. 2014. Bicarbonate uptake via anion exchange protein is the main mechanism of inorganic carbon acquisition by the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae) under variable pH. Journal of Phycology 50: 998–1008. DOI: 10.1111/jpy.12247.
- Ferriss B.E., Reum J.C.P., McDonald P.S., Farrell D.M. & Harvey C.J. 2016. Evaluating trophic and non-trophic effects of shellfish aquaculture in a coastal estuarine foodweb. ICES Journal of Marine Science 73: 429–440. DOI: 10.1093/icesjms/fsv173.
- Food and Agriculture Organization. 2016. The state of world fisheries and aquaculture 2016. Contributing to food security and nutrition for all. FAO, Rome, Italy. 200 pp.
- Forsyth R.G., Oldham M.J. & Schueler F.W. 2008. Mollusca, Gastropoda, Ellobiidae, Carychium minimum, and Ferussaciidae, Cecilioides acicula: distribution extension and first provincial records of two introduced land snails in Ontario, Canada. Check List 4: 449–452. DOI: 10.15560/4.4.449.
- Frieder C.A., Gonzalez J.P., Bockmon E.E., Navarro M.O. & Levin L.A. 2014. Can variable pH and low oxygen moderate ocean acidification outcomes for mussel larvae? Global Change Biology 20: 754–764. DOI: 10.1111/gcb.12485.
- Gazeau F., Parker L.M., Comeau S., Gattuso J.-P., O’Connor W.A., Martin S., Pörtner H.O. & Ross P.M. 2013. Impacts of ocean acidification on marine shelled molluscs. Marine Biology 160: 2207–2245. DOI: 10.1007/s00227-013-2219-3.
- Granada L., Sousa N., Lopes S. & Lemos M.F.L. 2016. Is integrated multitrophic aquaculture the solution to the sectors’ major challenges? – a review. Reviews in Aquaculture 6: 1–18. DOI: 10.1111/raq.12093.
- Green M.A., Waldbusser G.G., Hubazc L., Cathcart E. & Hall J. 2013. Carbonate mineral saturation state as the recruitment cue for settling bivalves in marine muds. Estuaries and Coasts 36: 18–27. DOI: 10.1007/s12237-012-9549-0.
- Greiner C.M., Klinger T., Ruesink J.L., Barber J.S. & Horwith M. 2018. Habitat effects of macrophytes and shell on carbonate chemistry and juvenile clam recruitment, survival, and growth. Journal of Experimental Marine Biology and Ecology 509: 8–15. DOI: 10.1016/j.jembe.2018.08.006.
- Groner M.L., Burge C.A., Cox R., Rivlin N.D., Turner M., Van Alstyne K.L., Wyllie-Echeverria S., Bucci J., Staudigel P. & Friedman C.S. 2018. Oysters and eelgrass: potential partners in a high pCO2 ocean. Ecology 99: 1802–1814. DOI: 10.6084/m9.figshare.6182522.
- Gurgel C.F.D., Norris J.N., Schmidt W.E., Le H.N. & Fredericq S. 2018. Systematics of the Gracilariales (Rhodophyta) including new subfamilies, tribes, subgenera, and two new genera, Agarophyton gen. nov. and Crassa gen. nov. Phytotaxa 374: 1–23. DOI: 10.11646/phytotaxa.374.1.1.
- Han T., Shi R., Qi Z., Huang H., Liang Q. & Liu H. 2017. Interactive effects of oyster and seaweed on seawater dissolved inorganic carbon systems: implications for integrated multi-trophic aquaculture. Aquaculture Environment Interactions 9: 469–478. DOI: 10.3354/aei00246.
- Harley C.D.G., Anderson K.M., Demes K.W., Jorve J.P., Kordas R.L., Coyle T.A. & Graham M.H. 2012. Effects of climate change on global seaweed communities. Journal of Phycology 48: 1064–1078. DOI: 10.1111/j.1529-8817.2012.01224.x.
- Harrington L., Fabricius K., Eaglesham G. & Negri A. 2005. Synergistic effects of diuron and sedimentation on photosynthesis and survival of crustose coralline algae. Marine Pollution Bulletin 51: 415–527. DOI: 10.1016/j.marpolbul.2004.10.042.
- Harrison P.J. & Hurd C.L. 2001. Nutrient physiology of seaweeds: application of concepts to aquaculture. Cahiers De Biologie Marine 42: 71–82.
- Harvey B., Soto D., Carolsfeld J., Beveridge M.C.M. & Bartley D.M. 2017. Planning for aquaculture diversification: the importance of climate change and other drivers. FAO Fisheries and Aquaculture Proceedings No. 47, Rome, Italy. 166 pp.
- Häussermann V. & Försterra G. [Eds] 2009. Marine benthic fauna of Chilean Patagonia. World Color Chile, Santiago, Chile. 1000 pp.
- Hendriks I.E., Duarte C.M., Olsen Y.S., Steckbauer A., Ramajo L., Moore T.S., Trotter J.A. & MacCulloch M. 2015. Biological mechanisms supporting adaptation to ocean acidification in coastal ecosystems. Estuarine, Coastal and Shelf Science 152: A1–A8. DOI: 10.1016/j.ecss.2014.07.019.
- Hendriks I.E. Olsen Y.S., Ramajo L., Basso L., Steckbauer A., Moore T.S., Howard J. & Duarte C.M. 2014. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 11: 333–346. DOI: 10.5194/bg-11-333-2014.
- Hepburn C.D., Pritchard D.W., Cornwall C.E., McLeod R.J., Beardall J., Raven J.A. & Hurd C.L. 2011. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Global Change Biology 17: 2488–2497. DOI: 10.1111/j.1365-2486.2011.02411.x.
- Hoffmann A.J. & Santelices B. 1997. Flora marina de Chile central. Ediciones Universidad Catolica de Chile, Santiago, Chile. 434 pp.
- Hofmann G.E., Smith J.E., Johnson K.S., Send U., Levin L.A., Micheli F., Paytan A., Price N.N., Peterson B., Takeshita Y. et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6: e28983. DOI: 10.1371/journal.pone.0028983.
- Huo Y., Wu H., Chai Z., Xu S., Han F., Dong L. & He P. 2012. Bioremediation efficiency of Gracilaria verrucosa for an integrated multi-trophic aquaculture system with Pseudosciaena crocea in Xiangshan Harbor, China. Aquaculture 326-329: 99–105. DOI: 10.1016/j.aquaculture.2011.11.002.
- Hurd C.L. 2000. Water motion, marine macroalgal physiology, and production. Journal of Phycology 36: 453–472. DOI: 10.1046/j.1529-8817.2000.99139.x.
- Hurd C.L. 2015. Slow-flow habitats as refugia for coastal calcifiers from ocean acidification. Journal of Phycology 51: 599–605. DOI: 10.1111/jpy.12307.
- Hurd C.L., Harrison P.J., Bischof K. & Lobban C.S. 2014. Seaweed ecology and physiology, ed. 2. Cambridge University Press, Cambridge, UK. 562 pp.
- Instituto de Fomento Pesquero. 2019. CHONOS. http://chonos.ifop.cl/.
- Intergovernmental Panel on Climate Change. 2013. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, UK. 1535 pp.
- Jeffs A.G., Delorme N.J., Stanley J., Zamora L.N. & Sim-Smith C. 2018. Composition of beachcast material containing green-lipped mussel (Perna canaliculus) seed harvested for aquaculture in New Zealand. Aquaculture 488: 30–38. DOI: 10.1016/j.aquaculture.2018.01.024.
- Jiang Z., Li J., Qiao X., Wang G., Bian D., Jiang X., Liu Y., Huang D., Wang W. & Fang J. 2015. The budget of dissolved inorganic carbon in the shellfish and seaweed integrated mariculture area of Sanggou Bay, Shandong, China. Aquaculture 446: 167–174. DOI: 10.1016/j.aquaculture.2014.12.043.
- Keeley N.B., Forrest B., Hopkins G., Gillespie P., Knight B., Webb S., Clement D. & Gardner J. 2009. Sustainable aquaculture in New Zealand: review of the ecological effects of farming shellfish and other non-finfish species. Prepared for the Ministry of Fisheries. Cawthron Report No. 1476, Nelson, New Zealand. 174 pp.
- Koch M., Bowes G., Ross C. & Zhang X.H. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology 19: 103–132. DOI: 10.1111/j.1365-2486.2012.02791.x.
- Kotta J., Herkül K., Kotta I., Orav-Kotta H. & Lauringson V. 2009. Effects of the suspension feeding mussel Mytilus trossulus on a brackish water macroalgal and associated invertebrate community. Marine Ecology 30: 56–64. DOI: 10.1111/j.1439-0485.2009.00303.x.
- Krause-Jensen D., Marbà N., Sanz-Martin M., Hendriks I.E. Thyrring J., Carstensen J., Sejr M.K. & Duarte C.M. 2016. Long photoperiods sustain high pH in Arctic kelp forests. Science Advances 2: e1501938. DOI: 10.1126/sciadv.1501938.
- Kroeker K.J., Kordas R.L., Crim R.N., Hendriks I.E. Ramajo L., Singh G.S., Duarte C.M. & Gattuso J.-P. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biology 19: 1884–1896. DOI: 10.1111/gcb.12179.
- Krumhansl K.A., Okamoto D.K., Rassweiler A., Novak M., Bolton J.J., Cavanaugh K.C., Connell S.D., Johnson C.R., Konar B., Ling S.D. et al. 2016. Global patterns of kelp forest change over the past half-century. Proceedings of the National Academy of Sciences of the United States of America 113: 1385–1390. DOI: 10.1073/pnas.1606102113.
- Kurihara H. 2008. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Marine Ecology Progress Series 373: 275–284. DOI: 10.3354/meps07802.
- Ladner I., Su I., Wolfe S. & Oliver S. 2018. Economic feasibility of seaweed aquaculture in southern California. Masters thesis. University of California, Santa Barbara, USA. 88 pp.
- Laffoley D. & Grimsditch G. 2009. The management of natural coastal carbon sinks. IUCN, Gland, Switzerland. 53 pp.
- Leal P.P., Hurd C.L., Sander S.G., Armstrong E., Fernández P.A., Suhrhoff T.J. & Roleda M.Y. 2018. Copper pollution exacerbates the effects of ocean acidification and warming on kelp microscopic early life stages. Scientific Reports 8: 14763. DOI: 10.1038/s41598-018-32899-w.
- Lüning K. & Pang S. 2003. Mass cultivation of seaweeds: current aspects and approaches. Journal of Applied Phycology 15: 115–119. DOI: 10.1023/A:1023807503255.
- Maberly S.C., Raven J.A. & Johnston A.M. 1992. Discrimination between 12C and 13C by marine plants. Oecologia 91: 481–492. DOI: 10.1007/BF00650320.
- Madsen J.D., Chambers P.A., James W.F., Koch E.W. & Westlake D.F. 2001. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 444: 71–84. DOI: 10.1023/A:1017520800568.
- Mao Y., Yang H., Zhou Y., Ye N. & Fang J. 2009. Potential of the seaweed Gracilaria lemaneiformis for integrated multi-trophic aquaculture with scallop Chlamys farreri in North China. Journal of Applied Phycology 21: 649–656. DOI: 10.1007/s10811-008-9398-1.
- Marin F., Corstjens P., De Gaulejac B., de Vrind-De Jong E. & Westbroek P. 2000. Mucins and molluscan calcification. Molecular characterization of mucoperlin, a novel mucin-like protein from the nacreous shell layer of the fan mussel Pinna nobilis (Bivalvia, Pteriomorphia). Journal of Biological Chemistry 275: 20667–20675. DOI:https://0.1074/jbc.M003006200.
- Martin S., Rodolfo-Metalpa R., Ransome E., Rowley S., Buia M.C. & Gattuso J.-P. 2008. Effects of naturally acidified seawater on seagrass calcareous epibionts. Biological Letters 4: 689–692. DOI: 10.1098/rsbl.2008.0412.
- McCulloch M.T., Falter J., Trotter J.A. & Montagna P. 2012. Coral resilience to ocean acidification and global warming through pH up-regulation. Nature Climate Change 2: 623–627. DOI: 10.1038/NCLIMATE1473.
- Méléder V., Populus J., Guillaumont B., Perrot T. & Mouquet P. 2010. Predictive modelling of seabed habitats: case study of subtidal kelp forests on the coast of Brittany, France. Marine Biology 157: 1525–1541. DOI: 10.1007/s00227-010-1426-4.
- Mišurcová L. 2012. Chemical composition of seaweeds. In: Handbook of marine macroalgae: biotechnology and applied phycology (Ed. by S.-K. Kim), pp. 175–192. John Wiley & Sons Ltd., Chichester, UK.
- Müller R., Laepple T., Bartsch I. & Wiencke C. 2009. Impact of oceanic warming on the distribution of seaweeds in polar and cold-temperate waters. Botanica Marina 52: 617–638. DOI: 10.1515/BOT.2009.080.
- Navarro J.M., Duarte C., Manríquez P.H., Lardies M.A., Torres R., Acuña K., Vargas C.A. & Lagos N.A. 2016. Ocean warming and elevated carbon dioxide: multiple stressor impacts on juvenile mussels from southern Chile. ICES Journal of Marine Science 73: 764–771. DOI: 10.1093/icesjms/fsv249.
- Navarro J.M., Torres R., Acuña K., Duarte C. & Manriquez P.H. 2013. Impact of medium-term exposure to elevated pCO2 levels on the physiological energetics of the mussel Mytilus chilensis. Chemosphere 90: 1242–1248. DOI: 10.1016/j.chemosphere.2012.09.063.
- Newell R.I.E. 2007. A framework for developing “ecological carrying capacity” mathematical models for bivalve mollusc aquaculture. Bulletin of Fisheries Research Agency 19: 41–51.
- Onitsuka T., Takami H., Muraoka D., Matsumoto Y., Nakatsubo A., Kimura R., Ono T. & Nojiri Y. 2018. Effects of ocean acidification with pCO2 diurnal fluctuations on survival and larval shell formation of Ezo abalone, Haliotis discus hannai. Marine Environmental Research 134: 28–36. DOI: 10.1016/j.marenvres.2017.12.015.
- Pantoja S., Iriarte J.L. & Daneri G. 2011. Oceanography of the Chilean Patagonia. Continental Shelf Research 31: 149–153. DOI: 10.1016/j.csr.2010.10.013.
- Peterson C.H., Summerson H.C. & Duncan P.B. 1984. The influence of seagrass cover on population structure and individual growth rate of a suspension-feeding bivalve, Mercenaria mercenaria. Journal of Marine Research 42: 123–138. DOI: 10.1357/002224084788506194.
- Pettit L.R., Smart C.W., Hart M.B., Milazzo M. & Hall-Spencer J.M. 2015. Seaweed fails to prevent ocean acidification impact on foraminifera along a shallow-water CO2 gradient. Ecology and Evolution 5: 1784–1793. DOI: 10.1002/ece3.1475.
- Przeslawski R., Byrne M. & Mellin C. 2015. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Global Change Biology 21: 2122–2140. DOI: 10.1111/gcb.12833.
- Raven J.A., Giordano M., Beardall J. & Maberly S.C. 2012. Algal evolution in relation to atmospheric CO2: carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philosophical Transactions of the Royal Society B 367: 493–507. DOI: 10.1098/rstb.2011.0212.
- Resplandy L., Keeling R.F., Eddebbar Y., Brooks M.K., Wang R., Bopp L., Long M.C., Dunne J.P., Koeve W. & Oschlies A. 2018. Quantification of ocean heat uptake from changes in atmospheric O2 and CO2 composition. Nature 563: 105–108. DOI: 10.1038/s41586-018-0651-8.
- Ridler N., Wowchuk M., Robinson B., Barrington K., Chopin T., & Robinson S. etal. 2007. Integrated multi-trophic aquaculture (imta): A potential strategic choice for farmers. Aquaculture Economics & Management 11: 99–110.
- Roleda M.Y., Boyd P.W. & Hurd C.L. 2012. Before ocean acidification: calcifier chemistry lessons. Journal of Phycology 48: 840–843. DOI: 10.1111/j.1529-8817.2012.01195.x.
- Roleda M.Y. & Hurd C.L. 2019. Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia 58: 552–562. DOI: 10.1080/00318884.2019.1622920.
- Romo H., Alveal K. & Werlinger C. 2001. Growth of the commercial carragenophyte Sarcothalia crispata (Rhodophyta, Gigartinales) on suspended culture in central Chile. Journal of Applied Phycology 13: 229–234. DOI: 10.1023/A:1011173515578.
- Romo H., Avila M., Núñez M., Pérez R., Candia A. & Aroca G. 2006. Culture of Gigartina skottsbergii (Rhodophyta) in southern Chile. A pilot scale approach. Journal of Applied Phycology 18: 307–314. DOI: 10.1007/s10811-006-9026-x.
- Saderne V., Fietzek P., Aßmann S., Körtzinger A. & Hiebenthal C. 2015. Seagrass beds as ocean acidification refuges for mussels? High resolution measurements of pCO2 and O2 in a Zostera marina and Mytilus edulis mosaic habitat. Biogeosciences Discuss 12: 11423–11461. DOI: 10.5194/bgd-12-11423-2015.
- SeafoodSource. 2019. SeafoodSource. https://www.seafoodsource.com/; searched on 20 September 2001.
- Semesi I.S., Beer S. & Björk M. 2009. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Marine Ecology Progress Series 382: 41–47. DOI: 10.3354/meps07973.
- Servicio Nacional de Pesca y Acuicultura. 2018. Anuarios estadísticos de pesca. http://www.sernapesca.cl/?option=com_remository&Itemid=246&func=select&id=334; searched on 04 October 2018.
- Silva N. & Vargas C.A. 2014. Hypoxia in Chilean Patagonian fjords. Progress in Oceanography 129: 62–74. DOI: 10.1016/j.pocean.2014.05.016.
- Tang Q., Zhang J. & Fang J. 2011. Shellfish and seaweed mariculture increase atmospheric CO2 absorption by coastal ecosystems. Marine Ecology Progress Series 424: 97–104. DOI: 10.3354/meps08979.
- Torres R., Pantoja S., Harada N., Gonzalez H.E., Daneri G., Frangopulos M., Rutllant J., Duarte C.M., Rúiz-Halpern S., Mayol E. et al. 2011. Air-sea CO2 fluxes along the coast of Chile: from CO2 outgassing in central northern upwelling waters to CO2 uptake in southern Patagonian fjords. Journal of Geophysical Research: Oceans 116: C09006. DOI: 10.1029/2010JC006344.
- Troell M. 2009. Integrated marine and brackishwater aquaculture in tropical regions: research, implementation and prospects. In: Integrated mariculture: a global review (Ed. by D. Soto) FAO Fisheries and Aquaculture Technical Paper. No. 529, pp. 47–131. FAO, Rome, Italy.
- Troell M., Joyce A., Chopin T., Neori A., Buschmann A.H. & Fang J.-G. 2009. Ecological engineering in aquaculture — potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 297: 1–9. DOI: 10.1016/j.aquaculture.2009.09.010.
- Troell M., Naylor R.L., Metian M., Beveridge M.C.M., Tyedmers P.H., Folke C., Arrow K.J., Barrett S., Crépin A.-S., Ehrlich P.R., et al. 2014. Does aquaculture add resilience to the global food system? Proceedings of the National Academy of Sciences of the United States of America 111: 13257–13263. DOI: 10.1073/pnas.1404067111.
- Turan G. & Neori A. 2010. Intensive seaweed aquaculture: a potent solution against global warming. In: Seaweeds and their role in globally changing environments (Ed. by A. Israel, R. Einav & J. Seckbach), pp. 359–372. Springer, London, UK.
- Unsworth R.K.F., Collier C.J., Henderson G.M. & McKenzie L.J. 2012. Tropical seagrass meadows modify seawater carbon chemistry: implications for coral reefs impacted by ocean acidification. Environmental Research Letters 7: 24026. DOI: 10.1088/1748-9326/7/2/024026.
- Valle-Levinson A., Sarkar N., Sanay R., Soto D. & León-Muñoz J. 2007. Spatial structure of hydrography and flow in a Chilean fjord, Estuario Reloncaví. Estuaries and Coasts 30: 113–126. DOI: 10.1007/BF02782972.
- Varela D.A., Henríquez L.A., Fernández P.A., Leal P.P., Hernández-González M.C., Figueroa F.L. & Buschmann A.H. 2018. Photosynthesis and nitrogen uptake of the giant kelp Macrocystis pyrifera (Ochrophyta) grown close to salmon farms. Marine Environmental Research 135: 93–102. DOI: 10.1016/j.marenvres.2018.02.002.
- Vargas C.A., Contreras P.Y., Pérez C.A., Sobarzo M., Saldías G.S. & Salisbury J.E. 2016. Influences of riverine and upwelling waters on the coastal carbonate system off Central Chile, and their ocean acidification implications. Journal of Geophysical Research: Biogeosciences 121: 1468–1483. DOI: 10.1002/2015JG003213.
- Waldbusser G.G., Hales B., Langdon C.J., Haley B.A., Schrader P., Brunner E.L., Gray M.W., Miller C.A., Gimenez I. & Hutchinson G. 2015. Ocean acidification has multiple modes of action on bivalve larvae. PLoS ONE 10: e01283. DOI: 10.1371/journal.pone.0128376.
- Waldbusser G.G. & Salisbury J.E. 2014. Ocean acidification in the coastal zone from an organism’s perspective: multiple system parameters, frequency domains, and habitats. Annual Review of Marine Science 6: 221–247. DOI: 10.1146/annurev-marine-121211-172238.
- Westermeier R., Gómez I. & Rivera P. 1993. Suspended farming of Gracilaria chilensis (Rhodophyta, Gigartinales) at Cariquilda River, Maullín, Chile. Aquaculture 113: 215–229. DOI: 10.1016/0044-8486(93)90475-E.
- Westermeier R., Patiño D.J., Murúa P., Quintanilla J.C., Correa J., Buschmann A.H. & Barros I. 2012. A pilot-scale study of the vegetative propagation and suspended cultivation of the carrageenophyte alga Gigartina skottsbergii in southern Chile. Journal of Applied Phycology 24: 11–20. DOI: 10.1007/s10811-010-9640-5.
- Wiedemeyer W.L. & Schwamborn R. 1996. Detritus derived from eelgrass and macroalgae as potential carbon source for Mytilus edulis in Kiel Fjord, Germany: a preliminary carbon isotopic study. Helgoländer Meeresuntersuchungen 50: 409–413. DOI: 10.1007/BF02367112.
- Willett W., Rockström J., Loken B., Springmann M., Lang T., Vermeulen S., Garnett T., Tilman D., DeClerck F., Wood A. et al. 2019. Food in the Anthropocene: the EAT–lancet Commission on healthy diets from sustainable food systems. Lancet Communications 393: 447–492. DOI: 10.1016/S0140-6736(18)31788-4.
- Xiao X., Agusti S., Lin F., Li K., Pan Y., Yu Y., Zheng Y., Wu J. & Duarte C.M. 2017. Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Scientific Reports 7: 46613. DOI: 10.1038/srep46613.
- Xu Q., Gao F. & Yang H. 2016. Importance of kelp-derived organic carbon to the scallop Chlamys farreri in an integrated multi-trophic aquaculture system. Chinese Journal of Oceanology and Limnology 34: 322–329. DOI: 10.1007/s00343-015-4332-2.

