Abstract
Background
Reduced activity of the sucrase-isomaltase (SI) enzyme can cause gastrointestinal symptoms. Biochemical measurement of SI activity in small intestinal biopsies is presently considered the gold standard for the diagnosis of SI deficiency, but this invasive test is not suitable as a routine diagnostic tool.
Aim
To evaluate a 13C-sucrose-breath test (13CSBT) as a diagnostic tool for SI deficiency in an adult population.
Methods
13CSBT results were compared to sucrase activity measured in duodenal biopsies.
Results
Forty patients with gastrointestinal symptoms were included in the study, 4 of whom had celiac disease and the rest (n = 36) had normal histological findings. Nine patients (22.5%) had low sucrase activity measured using duodenal biopsies. No correlation was observed between enzymatic sucrase activity and the 13CSBT results. The 13CSBT-curves for the celiac patients versus patients with normal duodenal histology demonstrated that the patients with celiac disease were within the lower range of the distribution.
Conclusion
We observed a mismatch between the 13CSBT results and the biochemically measured sucrase activity, suggesting that SI activity is not uniformly distributed throughout the small intestines. This methodological discrepancy should be acknowledged when diagnosing SI deficiency.
Introduction
Sucrose, commonly known as table sugar, is a disaccharide made up of the two monosaccharides, glucose and fructose. Digestion of foods containing sugar is dependent on the enzyme sucrase-isomaltase (SI), which is the only enzyme that hydrolyses sucrose [Citation1]. SI is a membrane-bound enzyme complex located on enterocytes at the tip of the villi of the proximal small intestine [Citation2]. Individuals lacking the SI enzyme or having reduced sucrase activity will not be able to properly digest sucrose, and this commonly leads to gastrointestinal (GI) symptoms [Citation2]. SI deficiency (SID) can be either primary or secondary, and the prevalence is unknown.
The current gold standard for the diagnosis of SID is low or absent sucrase activity measured in biopsies from small intestinal mucosa obtained from gastroscopy, in combination with normal histology to exclude secondary deficiency [Citation3,Citation4]. Of note, measurement of sucrase activity in biopsy samples is not a common routine test in current clinical practice in most countries. In addition, the analysis is resource demanding, and requires a laboratory with specialist expertise and equipment [Citation2].
A 13C-labeled sucrose breath test (13CSBT) has been suggested as a specific non-invasive confirmatory test for SID [Citation5–7], however the 13CSBT has not been formally validated for use in a clinical setting in an adult population [Citation8]. Hence, we aimed to establish a 13CSBT to investigate whether this test is: (1) correlated to a biochemical assay of sucrase activity and (2) able to distinguish pathological small intestinal mucosa from normal small intestinal mucosa.
Materials and methods
Study design
Patients were consecutively included on the day of their gastroscopic examination. After inclusion, all patients were contacted by a study nurse, to schedule the breath test assessment, which was performed at most four weeks after obtaining their biopsies. Height and weight were measured on the day of the breath test.
Participants
Patients were consecutively recruited into the study as they were referred to the outpatient clinic for routine gastroscopic examination at Lovisenberg Diaconal Hospital, Oslo, Norway, between August 2022 and March 2023. Eligibility was evaluated by the gastroenterologist (JV) before the gastroscopy, and patients fulfilling the inclusion criteria were invited to participate. All candidates interested in participating received more information and signed the informed consent form. Inclusion criteria were referral for gastroscopic examination with a suspected GI disorder and signed informed consent, and there were no exclusion criteria except for an unwillingness or inability to provide written informed consent.
Ethics
The study was conducted according to the guidelines specified in the Declaration of Helsinki. The Regional Committee for Medical and Health Research Ethics of South-Eastern Norway (2021/338236) approved the project, and the trial was registered at clinicaltrials.gov (NCT05159115). All subjects gave written informed consent.
Breath test performance
All subjects were asked to fast from midnight the day before the breath test until after it was completed. Required medications were allowed with water. After overnight fasting, patients met at the study ward between 08 and 09 am, and a baseline breath sample was collected for comparison with the timed breath samples. Breath samples were collected by breathing through a straw into a sterile glass container (12 mL exetainer® Breath Vial. Prod; Labco Limited, Ceredigion SA48 7 HH, United Kingdom). Two samples were collected for each time point. After the baseline sample, 10 mg uniformly labeled 13C-sucrose (D-sucrose, Cambridge Isotope Laboratories, Inc.) was given, using 190 mg unlabeled sucrose (Sucrose EMPROVE® ESSENTIAL Ph, Sigma Aldrich) as a carrier. The sucrose-solution was dissolved in 250 ml of water. Breath samples were collected 15 min after the sucrose load, and every 15 min thereafter for 240 min.
Analysis of 13CO2 in breath samples and calculations of raw data
13CO2 in breath samples were quantified in duplicates for each 15th min interval and were measured using a 13CO2 Isotope Ratio Mass Spectrometer (ABCA2 13Carbon Analyzer, Sercon Ltd, United Kingdom). Raw breath enrichment data were calculated as the mean of the two δ-values obtained for each time point by mass-spectrometric analysis. Mean raw values were then converted to %13C and further combined with anthropometric data (height and weight) to compute 13C excretion rates as the percentage of 13C dose per hour, as well as the cumulative percentage of administered dose of 13C recovered over time, according to equations by Ghoos et al. [Citation9]. Correcting raw data for body surface area allows direct comparison of data between individuals. The shape of the %13C dose/h-curve reflects the rate at which the process occurs (delayed, accelerated with/without lag phase) and the %13C cumulative dose is derived numerically from the %13C dose/h-data and informs about the global process [Citation9]. The variables used for interpreting breath test data and assessing the 13CSBT response included total area under the curve (AUC) and cumulative dose at 90 min (CD90). Total AUC (i.e. for the whole test time period) was calculated by the statistical program for each % 13 C dose/hour curve. In addition, the % cumulative dose at 90 min (CD90) reflects the digestion of sucrose in the small intestinal phase, and was calculated directly according to equations by Ghoos et al. [Citation9].
Biopsy sampling and enzymatic analysis
Routine upper gastrointestinal endoscopy was performed after overnight fasting. Three pinch biopsies were collected from the distal part of the duodenum. The biopsies were collected with freshly opened biopsy forceps, placed in a fresh (sterile), dry sample tube and immediately placed on ice before being stored in a minus 80 °C freezer until analysis. Thereafter, two pinch biopsies were collected and placed in formalin as part of standard clinical diagnostic protocol for the evaluation of biopsy morphometry related to diagnosis of celiac disease.
SI activity was measured by an established clinical speciality diagnostic and translational research laboratory at Orlando Health, Orlando, Florida, USA, using a modified Dahlqvist method [Citation3,Citation10]. The biopsies were homogenized, and by using sucrose substrate, the glucose production was measured by glucose oxidase. Total protein concentration was measured using Pierce BCA protein assay kit (Pierce, Thermo Scientific, USA). Specific enzyme activity was expressed as units (U), defined as micromoles of glucose released per minute per gram of mucosal protein at 37 °C. The cut off values were based on the analysis of over 10,000 assays using standard protocols [Citation11]. Low or abnormal enzyme activity was defined by values below the 3rd percentile in the normal reference range, corresponding to sucrase values <28.7 U.
Statistical analyses
A power calculation was performed to estimate the necessary number of subjects. Based on preliminary results suggesting that 35% of patients with irritable bowel syndrome have SI deficiency [Citation12], one is 95% likely to find between 8 and 21 patients with SI deficiency when examining 40 patients. Assuming 80% concordance between the two methods (correct proportion of successes), one would need 12 positive cases. Thus, if 40 individuals are included, the study is sufficiently powered (alpha = 5% and beta = 20%, using McNemar’s test of concordance).
AUC values between groups were assessed with Mann-Whitney test as these variables were skewed and the sample size was limited. Correlations between sucrase activity and variables from the 13CSBT were computed using Spearman correlation coefficients. The results are expressed as median (min-max) for non-parametric data. P-values <0.05 were considered statistically significant. All analyses were considered exploratory so no correction for multiple testing was done. All analyses and graphical illustrations were made in GraphPad Prism version 10 (Dotmatics, Boston, USA).
Results
Characteristics of study subjects
Fifty-one eligible patients were referred for gastroscopy for examination of upper gastrointestinal symptoms during the inclusion period. Eleven patients withdrew after the gastroscopic examination and did not complete the breath test. Forty patients (25 women and 15 men) completed the breath test and were included in the analyses. Baseline characteristics are shown in . Thirty-six patients had normal small intestinal endoscopy without pathological findings. Four patients had untreated celiac disease with pathological small intestinal histology, seen as Marsh types 3 A, 3 C, 3B and 3B-C, respectively.
Table 1. Baseline characteristics of the 40 patients included in the study.
Breath test results versus enzyme activity measured in small intestinal biopsies
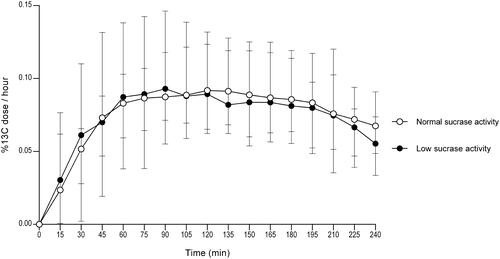
Nine patients (9/40; 22.5%) had low sucrase activity according to enzymatic analysis. The breath test curve for the group with low sucrase activity according to small intestinal biopsy analysis was similar to the curve for subjects with normal enzyme activity (). Our data did not reveal statistically significant differences in the breath test results when comparing the group with normal enzyme activity to the group with low enzyme activity.
Figure 1. Breath test results showing 13C-labeled CO2 in exhaled breath for nine patients with low sucrase activity measured in duodenal biopsy samples versus 31 patients with normal sucrase activity measured in duodenal biopsy samples. Error bars represent median with range.
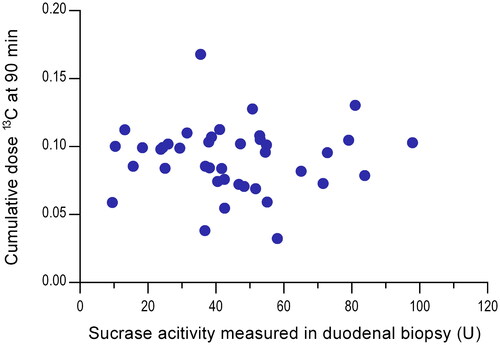
There was no correlation between enzymatic sucrase activity measured in the small intestinal biopsies and individual AUC values (r = 0.007, p = 0.97) or CD90 values (r= −0.067, p = 0.68; ).
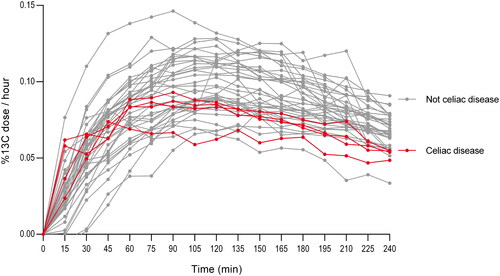
Breath test result comparison of patients with celiac disease versus patients with normal histological findings
To investigate whether the 13CSBT was able to separate a pathological small intestinal mucosa from a normal small intestinal mucosa, we compared subjects with celiac disease (n = 4) to those with normal histological findings (n = 36). The breath test curves for the celiac patients versus the patients with normal small intestinal histology (), demonstrated that the patients with celiac disease were within the lower range of the distribution of the individuals. No statistically significant difference was seen when comparing the AUC (1.12 (0.89–1.12) vs 1.29 (0.73–1.76), p = 0.21) or CD90 (0.09 (0.08–0.10) vs 0.10 (0.03–0.17), p = 0.91) between the celiac patients and the patients with normal small intestinal histology, respectively.
Discussion
SID is suggested to be present in a considerable proportion of patients with GI symptoms, either due to a genetic variant or an acquired, secondary cause [Citation2]. Due to the lack of a validated non-invasive diagnostic test for sucrase deficiency, the condition is not generally diagnosed in adults, not even patients with GI symptoms, and the prevalence is unknown. Appropriate and convenient diagnostic methods may improve treatment for many patients. Here, we have evaluated a non-invasive breath test with 13C-labeled sucrose compared to the ‘gold standard’ sucrase enzyme activity assay of distal duodenal biopsies obtained by endoscopy, and showed that the 13CSBT results did not correlate with enzymatic sucrase activity.
The lack of correlation between enzymatic sucrase activity and the 13CSBT results does not necessarily imply that that the 13CSBT is not capable of detecting reduced SI activity, but may rather reflect an uneven expression of SI enzyme in the gut. SI activity may be variable throughout the small intestines, hence a biopsy might not provide the correct diagnosis. As analysis of biopsy material is based on a few small samples from a very limited area from the longitudinal axis of the small intestines, it might not be representative of the enzymatic status of the entire length of the small intestines. In addition, it is more likely the situation to assume that the reserve capacity is substantial, unless one has severe/genetic SID, which is a rare condition [Citation2].
Our findings corroborate with previously reports. A study investigating the use of 13CSBT for assessment of environmental enteropathy in 24 African adults demonstrated that the breath test results did not correlate with sucrase activity measured in duodenal biopsy samples, although the breath test generated clear enrichment of 13CO2 in breath samples and clearly indicated the presence of sucrase activity [Citation7]. Another study in 20 children (10 with congenital SID and 10 controls) previously reported the 13CSBT to be an accurate and specific non-invasive confirmatory test for congenital SID in children [Citation13]. The study compared enzyme activity in duodenal biopsy samples with 13CSBT similar to the method used in our study [Citation13]. In contrast to our findings, a rat model investigating a 13CSBT for small intestinal damage, reported that biochemical sucrase activity is correlated with CD90 13CSBT results [Citation14]. Of note, they measured the SI activity in jejunal biopsies, whereas our samples were collected from the duodenum. Although a rat model might not be representative for the human gut, this may implicate that jejunal samples express more SI enzymes than duodenal samples and thus would contribute more to the overall gut SI activity.
We found no differences between breath test curves for the celiac patients versus the patients with normal small intestinal histology. However, the celiac curves were within the lower range of the population and two out of four celiac patients had biochemically sucrase deficiency, suggesting that patients with pathological small intestinal mucosa might be prone to secondary SID. As only four patients had celiac disease, the group is too small to draw any definitive conclusions. Our study results warrant further investigation in a larger group of patients with GI-diseases affecting villi height in the small intestines.
Our dose of 10 mg 13C-labelled sucrose is lower than what has been reported by previous studies, with doses ranging from 20 to 50 mg or 0,3 mg/kg body weight [Citation7,Citation13,Citation15]. As little evidence exists on the most appropriate dose of labelled 13C-sucrose, it can be questioned whether we used a high enough dose to produce an optimal breath test curve of expired 13CO2. Of note,13C-labeled sucrose is expensive, hence it is beneficial to use a dose as low as possible and our results suggests that the current dose was sufficient to produce a clear enrichment of 13CO2 in the breath samples.
In conclusion, we observed a mismatch between the 13CSBT results and the biochemically measured sucrase activity, suggesting that SI activity is not uniformly distributed throughout the small intestines. This important methodological discrepancy should be taken into consideration when diagnosing SID in future studies as well as in current clinical practice.
Authors’ contributions
HFD, MCS, VS and JV planned and designed the study. JV and HFD conducted the research. DC performed the enzymatic assay of small intestinal biopsies. HFD performed the data analyses and wrote the manuscript. JV contributed to data interpretation and critical revision of the manuscript. All authors reviewed and approved the final manuscript.
Acknowledgements
We thank the volunteer patients for their participation in this study. We thank Gunn Helen Malmstøm, Jennifer Fiennes and Anita Tollisen for help performing the breath tests, Ana Urzua Riquelme for administrative help, and Arne G. Røseth for help with the inclusion process.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Andersen MK, Skotte L, Jørsboe E, et al. Loss of sucrase-isomaltase function increases acetate levels and improves metabolic health in greenlandic cohorts. Gastroenterology. 2022;162(4):1171–1182.e3. doi: 10.1053/j.gastro.2021.12.236.
- Chey DWCB, Lembo A, Patel DB, et al. Congenital sucrase-isomaltase deficiency: what, when, and how? Gastroenterol & Hepatol. 2020;16(10):1–11.
- Dahlqvist A. Assay of intestinal disaccharidases. Scand J Clin Lab Invest. 1984;44(2):169–172. doi: 10.3109/00365518409161400.
- Karnsakul W, Luginbuehl U, Hahn D, et al. Disaccharidase activities in dyspeptic children: biochemical and molecular investigations of maltase-glucoamylase activity. J Pediatr Gastroenterol Nutr. 2002;35(4):551–556. doi: 10.1097/00005176-200210000-00017.
- Ritchie BK, Brewster DR, Davidson GP, et al. 13C-sucrose breath test: novel use of a noninvasive biomarker of environmental gut health. Pediatrics. 2009;124(2):620–626. doi: 10.1542/peds.2008-2257.
- Wardill HR, Bowen JM, Gibson RJ. Biomarkers of small intestinal mucosal damage induced by chemotherapy: an emerging role for the 13C sucrose breath test. J Support Oncol. 2013;11(2):61–67. doi: 10.1016/j.suponc.2012.06.004.
- Schillinger RJ, Mwakamui S, Mulenga C, et al. 13)C-sucrose breath test for the non-invasive assessment of environmental enteropathy in Zambian adults. Front Med (Lausanne). 2022;9:904339. (doi: 10.3389/fmed.2022.904339.
- Robayo-Torres CC, Diaz-Sotomayor M, Hamaker BR, et al. 13C-labeled-starch breath test in congenital sucrase-isomaltase deficiency. J Pediatr Gastroenterol Nutr. 2018;66(Suppl 3):61–64.
- Ghoos Y, Geypens B, Maes B, et al. Breath tests in gastric emptying and transit studies: technical aspects of 13CO2-breath tests. Progress in understanding and management of gastro-intestinal motility disorders. Belgium: Katholieke Universiteit Leuven ; 1993; p. 169–180.
- Dahlqvist A, Nordström C. The distribution of disaccharidase activities in the villi and crypts of the small-intestinal mucosa. Biochim Biophys Acta. 1966;113(3):624–626. doi: 10.1016/s0926-6593(66)80024-3.
- Deb C, Campion S, Derrick V, et al. Sucrase-isomaltase gene variants in patients with abnormal sucrase activity and functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2021;72(1):29–35. doi: 10.1097/MPG.0000000000002852.
- Kim SB, Calmet FH, Garrido J, et al. Sucrase-isomaltase deficiency as a potential masquerader in irritable bowel syndrome. Dig Dis Sci. 2020;65(2):534–540. doi: 10.1007/s10620-019-05780-7.
- Robayo-Torres CC, Opekun AR, Quezada-Calvillo R, et al. 13C-breath tests for sucrose digestion in congenital sucrase isomaltase-deficient and sacrosidase-supplemented patients. J Pediatr Gastroenterol Nutr. 2009;48(4):412–418. doi: 10.1097/mpg.0b013e318180cd09.
- Tooley KL, Howarth GS, Lymn KA, et al. Optimization of the non-invasive 13C-sucrose breath test in a rat model of methotrexate-induced mucositis. Cancer Chemother Pharmacol. 2010;65(5):913–921. doi: 10.1007/s00280-009-1098-2.
- Opekun AR, Balesh AM, Shelby HT. Use of the biphasic (13)C-sucrose/glucose breath test to assess sucrose maldigestion in adults with functional bowel disorders. Biomed Res Int. 2016;2016:7952891. doi: 10.1155/2016/7952891.