Abstract
Background and aims
Therapeutic drug monitoring (TDM) may optimize biologic and thiopurine therapies in inflammatory bowel disease (IBD). The study aimed to investigate implementation and utilization of TDM in Scandinavia.
Methods
A web-based questionnaire on the use of TDM was distributed to Scandinavian gastroenterologists via the national societies.
Results
In total, 297 IBD physicians prescribing biologic therapies, equally distributed between community and university hospitals, were included (response rate 42%) (Norway 118 (40%), Denmark 86 (29%), Sweden 50 (17%), Finland 33 (11%), Iceland 10 (3%)). Overall, TDM was applied during biologic therapies by 87%, and for TNF-inhibitors >90%. Among the users, reactive and proactive TDM were utilized by 90% and 63%, respectively. Danish physicians were significantly less inclined to use TDM compared to other Scandinavian countries; (58% vs 98%); OR 0.03 [0.01–0.09], p < 0.001). Reactive TDM was commonly applied at primary (74%) and secondary (99%) treatment failure. Proactive TDM was used by 80% during maintenance therapy and 56% during induction and more commonly utilized in Norway (p < 0.001), and by physicians managing >10 IBD patients/week (p = 0.005). TDM scenarios were interpreted in accord with available evidence but with discrepancies for proactive TDM. The main barriers to TDM were lack of guidelines (51%) and time lag between sampling and results (49%). TDM of thiopurines was routinely used by 87%.
Conclusion
TDM of biologic and thiopurine therapies has been broadly implemented into clinical practice in Scandinavia. However, physicians call for TDM guidelines detailing indications and interpretations of test results along with improved test response times.
Introduction
Treatment of patients with inflammatory bowel disease (IBD) often involves biologics (tumour necrosis factor (TNF) inhibitors, integrin inhibitors, interleukin (IL)-12/23-inhibitors) or conventional immunosuppressives, notably thiopurines (azathioprine or mercaptopurine) [Citation1,Citation2]. Unfortunately, optimal efficacy of these agents is not universal and treatment failure occurs in a substantial proportion along with failure to attain mucosal or histological healing and key patient reported outcomes [Citation3,Citation4]. As there is a limited number of pharmaceutical options available, and because efficacy tends to decline when switching agent, it is desirable to optimize the treatment regimen and to rationally switch between agents, if needed [Citation5,Citation6]. Therapeutic drug monitoring (TDM) includes measurements of circulating drug, key metabolites, and anti-drug antibodies (ADAb) and has been introduced as a clinical tool to improve efficacy, safety, and cost-effectiveness [Citation7–10]. Hence, proactive TDM is used in quiescent disease to prevent disease worsening, and reactive TDM is used in primary non-response or secondary loss of response to treatment [Citation11,Citation12].
Over recent years, TDM has been introduced into clinical practice, but there is limited knowledge on how TDM has been taken up by physicians and implemented in patient care. Previous surveys are few and hampered by small sample sizes, dating back to when few agents were available and knowledge of TDM was sparse. Also, they originate from health care systems and physician populations that cannot be extrapolated to current practice in developed countries [Citation13–17]. While reactive TDM of biologics is now broadly recommended by both national and international IBD societies and by expert guidelines [Citation18–25], proactive TDM has generally not been endorsed for routine use in all patients [Citation19,Citation26]. However, recent clinical trials not yet incorporated into guidelines have shown positive results of proactive TDM [Citation27–29]. TDM during thiopurine therapy is broadly recommended both before initiation of therapy by measurements of thiopurine S-methyltransferase enzyme activity (TPMT) and during treatment by measurements of thiopurine metabolites (6-thioguanine nucleotide [6-TGN] and 6-methylmercaptopurine [6-MMP]) [Citation19,Citation30]. It is our impression that despite available TDM guidelines and a vast amount of publications, clinical use of TDM is still controversial among clinicians.
This was the first study among Scandinavian gastroenterologists evaluating the use of TDM in five developed countries with high prevalence of IBD. The aim of this study was to investigate how TDM has been taken up by physicians, application in everyday clinical practice, and barriers restricting implementation.
Material and methods
Study design
This web-based questionnaire survey on TDM for biologic and thiopurine therapies was carried out from January to September 2021. Invitations were distributed via e-mail sent from the national gastroenterology- and IBD societies in the five Scandinavian countries (Norway, Denmark, Sweden, Finland, and Iceland) and announced on relevant society- and IBD websites and in newsletters. Three reminders at 2 weeks intervals were sent out. Responders were excluded if not gastroenterology consultants or trainees, not involved in biologic treatment of IBD, or had incomplete survey responses which was defined as not having answered to questions concerning TDM of biologics. Data were collected and managed using REDCap electronic data capture tools hosted at Herlev Hospital, Denmark.
TDM questionnaire
The survey consisted of dynamic categorical multiple-choice questions comprising the following sections: physician demographics, IBD cohort characteristics, TDM for biologic therapies, TDM for thiopurine therapies, TDM and combination therapy, barriers towards TDM, and awareness of TDM guidelines. Furthermore, participants were introduced to a variety of theoretical TDM scenarios exploring interpretation of pro- and reactive TDM test results of biologic therapies. The questionnaire was reviewed by the study group to ensure validity and piloted within the study group before dissemination.
Statistical analyses
Data were analyzed using descriptive statistics. Comparisons between groups were evaluated using Fisher’s exact test for categorical variables. Associations between utilization of TDM and pre-chosen physician characteristics (country, health region, practice setting, grade, specialist experience, trainee experience, hospital catchment area, size of IBD cohort, and contacts with IBD patients/week) were assessed by bivariate and multiple logistic regression models. P-values <.05 were considered statistically significant. Data were analyzed using Stata v16 (StataCorp).
Ethical statement
The survey was registered and approved by the National Data Protection Authorities according to national legislations. All responses were anonymous and no specific patient-related information was collected.
Results
Study population
The survey was distributed to approximately 900 gastroenterology consultants and gastroenterology trainees of whom 377 (42%) responded. The study population comprised 297 physicians who handled IBD patients on biologic therapies and with complete TDM survey responses (Norway 118 (40%), Denmark 86 (29%), Sweden 50 (17%), Finland 33 (11%), Iceland 10 (3%) (). The majority (85%) were consultant gastroenterologists, 51% were employed at university hospitals, and 46% at community hospitals. Characteristics of responders are detailed in .
Figure 1. Flowchart of study population. *Answers to questions regarding TDM of biologic agents were lacking.
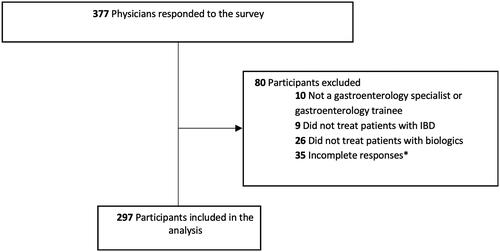
Table 1. Demographic and clinical characteristics of study participants (n = 297).
TDM of biological therapies
Characteristics and patterns of TDM utilization
Overall, 257 of 297 (87%) IBD physicians used TDM during biologic therapies. Indications for TDM were to improve efficacy (70%), aid treatment decisions (62%), improve safety (36%), and secure cost-effectiveness (32%). TDM was applied for all biologic agents, with the highest utilization rate (>90%) for infliximab and adalimumab ().
Table 2. Use of reactive and proactive TDM across different biologic drugs.
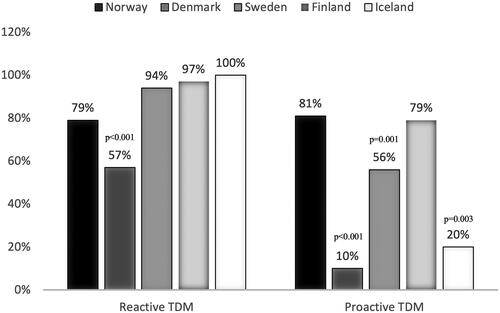
Among physicians using TDM, 231 (90%) used reactive TDM and 161 (63%) used proactive TDM. Combined pro- and reactive TDM was applied by 53%. Reactive TDM was applied at primary non-response (74%) and secondary treatment failure (99%); most commonly for infliximab and adalimumab, followed by vedolizumab, ustekinumab, and golimumab (). Proactive TDM was used by 80% during maintenance therapy and 56% during induction. In line with reactive TDM, proactive TDM was most frequently applied for infliximab and adalimumab, followed by vedolizumab, ustekinumab, and golimumab (). Physicians generally applied proactive TDM measurements once (91%) or twice a year (59%). In addition to pro- and reactive TDM, measurements of drug concentrations and ADAb levels were also applied in cases of side-effects by 53%, at re-initiation of therapy after a drug-holiday by 39%, and when considering discontinuation of biologic therapies by 30%. Utilization patterns across the Scandinavian countries are shown in .
Figure 2. The use of reactive versus proactive TDM of biologic agents among physicians in Scandinavia. Distribution of the current use of reactive and proactive TDM in clinical practice in Norway, Denmark, Sweden, Finland, and Iceland presented as frequencies (%). P-values are generated from multivariate logistic regression models, adjusted for country, employment at university hospital, size of hospital catchment area, seniority, and number of IBD contacts per week.
Among physicians who used TDM in clinical practice, 66% reported awareness of TDM guidelines or international expert recommendations. Local guidelines were available for reactive and proactive TDM for 28% and 34%, respectively.
Interpretation of TDM test results in clinical practice
To address how TDM was utilized by clinicians, we investigated the interpretation of various theoretical clinical case scenarios detailed in Supplementary Tables 1–2. For reactive TDM (Supplementary Table 1), where a patient per definition had objectively verified treatment failure, and in case of therapeutic drug levels and not detectable ADAb, 48% would switch out of biologic class. Other strategies were applied by a minority, for example, switch within biologic class (17%), dose intensify on current agent (16%), or optimize concomitant immunosuppression (13%). At treatment failure in the presence of ADAb, with sub-therapeutic drug levels, physicians preferably switched within biologic drug class (34%), and with some preferring to dose intensify (18%) or optimize concomitant immunosuppressive therapy (10%), and with 22% reporting that their strategy depended on ADAb levels. At treatment failure presenting with sub-therapeutic drug level and no ADAb, 99% would dose intensify the biologic agent.
Proactive TDM at quiescent disease without objective findings of active disease and presenting with therapeutic drug level, 92% would continue current treatment without any changes (Supplementary Table 2). Therapeutic drug level presenting with concurrent detection of ADAb would initiate optimization of concomitant immunosuppressive therapy among 30%, 10% would discontinue current treatment, and 17% would continue without any therapeutic changes. However, 37% would base their strategy depending on ADAb levels. Sub-therapeutic drug level without ADAb would lead to dose intensification for about two thirds of participants (63%) while the remaining generally would continue an unchanged regimen (29%). Sub-therapeutic drug level in the presence of ADAb was handled inconsistently by physicians; some of which would base their actions depending on ADAb levels (39%), discontinue current treatment (22%), dose intensify the current regimen (18%), or optimize concomitant immunosuppressive therapy (18%). Of note, 87% would reduce dosing at supra-therapeutic drug levels (Supplementary Table 2).
Factors affecting use of TDM
TDM as an overall approach was significantly less used in Denmark as compared to all other countries (58% vs 98%; OR 0.03 [0.01–0.09], p < .001) (Supplementary Table 3a). In particular, TDM was used to a lesser extent outside the Capital Region of Copenhagen (OR 0.15 [0.05–0.46], p = .001) (Supplementary Table 3 b). Similar results were observed for reactive TDM (Supplementary Table 4a-b).
In the multiple regression model, physicians from Denmark (OR 0.02 [0.01–0.04], p < .001), Sweden (OR 0.22 [0.09–0.52], p = .001) and Iceland (OR 0.07 [0.01–0.40], p = .003) used proactive TDM less than Norway ( and ). Physicians managing more than 10 IBD patients per week were more inclined to use proactive TDM (OR 2.71 [1.34–5.48], p = .005) (). Notably, Danish physicians using proactive TDM were all employed in the Capital region of Copenhagen (n = 9, p < .001).
Table 3. Factors associated with use of proactive TDM of biologic agents (n = 297).
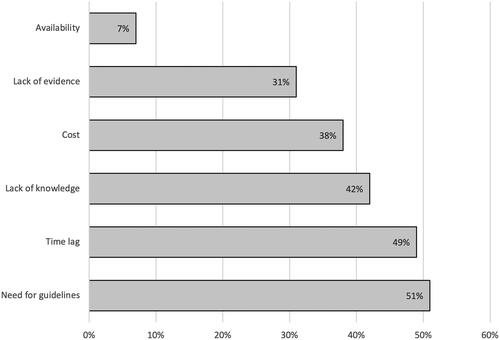
Barriers to TDM
Overall, 77% of physicians encountered barriers hindering implementation of TDM in clinical practice whereas 23% experienced no barriers at all. The main barriers were lack of knowledge of interpretation and indications for TDM alongside a need for TDM guidelines and time lag between sampling and test results (). There was no difference in commonly encountered barriers between countries except time lag which was more prominent in Finland (OR 3.43 [1.53–7.65], p = .004). In Denmark, where TDM was used to a lesser extent, the main barriers were lack of evidence (44%), lack of guidelines (39%), and lack of knowledge on proper interpretation of drug and ADAb measurements (36%).
TDM of thiopurine therapies
Indications and pattern of thiopurine usage
Thiopurines were used both as monotherapy (77%) and in combination with biologics (95%) –particular in combination with infliximab (98%) and adalimumab (74%) (Supplementary Table 5). Factors considered most important when prescribing thiopurines were age (80%), type of concomitant biologic agent (60%), and TPMT status (52%). A conventional “step-up” strategy with sequential progression from conventional biologics was generally preferred (Norway 23%, Denmark 58%, Sweden 73%, Finland 73%, and Iceland 71%). However, physicians from Norway more often used a ‘top-down’ strategy with early introduction of biologics as compared to all others (OR 6.56 [3.69–11.67], p < .001). Treatment duration of combination therapy was typically 1–2 years (55%); however, one-third had no restraints on the duration of thiopurine treatment (Supplementary Table 5).
Characteristics of TDM utilization
TDM of thiopurine therapy was utilized by most physicians (87%) and across all countries but somewhat less in Finland and Iceland (p < .001) (Supplementary Tables 5 and 6). Prior to initiation, about two third (65%) assessed TPMT geno- and/or phenotype and only 18% never assessed TPMT (Supplementary Table 5). During ongoing therapy, the vast majority (91%) assessed combined 6-TGN and 6-MMP metabolites and used both proactive and reactive TDM. Allopurinol was commonly prescribed for 6-MMP shunters by 70%. During combination therapy with biologics, thiopurine metabolites were measured by the majority (74%) and indications were to reduce risk of ADAb (63%) and improve efficacy of the biologic agent (61%). About half had access to local thiopurine TDM guidelines (47%) and knowledge of international guidelines and expert recommendations (56%) (Supplementary Table 5).
Barriers for TDM of thiopurine therapies
In all, 64% encountered barriers for TDM of thiopurines. As detailed in Supplementary Table 5, the most prominent were long waiting time for test results (39%) and an unmet need for guidelines (22%).
Discussion
This is the first multinational survey on how TDM of biologic and thiopurine therapies have been adopted and implemented in clinical practice by IBD specialists. In this survey covering five northern European countries, we show that TDM has become an integral part of everyday clinical practice to inform and guide therapeutic interventions. The study also identifies important national and regional differences in utilization patterns and interpretations of TDM test results, and points to key barriers that need to be addressed to attain higher adherence and more uniform utilization of TDM in clinical practice.
Reactive TDM at treatment failure was used by a vast majority of clinicians, for all biologic agents but predominantly TNF-inhibitors, and both during induction and maintenance phases. Notably, different representative scenarios revealed a high level of agreement on interpretation of reactive TDM results, revealing a high level of knowledge. Reactive TDM is well documented, and in line with available guidelines and consensus statements [Citation18–24],, there was agreement on switching out of class in case of therapeutic drug level (pharmacodynamic failure) and dose intensification at sub-therapeutic drug level without ADAb (non-immune pharmacokinetic failure). Furthermore, at presentation of neutralizing ADAb (immune-mediated pharmacokinetic failure), most would switch within class but with a notable minority preferring dose intensification or optimization of concomitant immunosuppression in effort to overcome ADAb – strategies that have all been proven valid [Citation31].
The introduction of TDM has been of great clinical impact in the care of IBD patients, but current guidelines mainly address reactive TDM, and recommendations for proactive TDM is very limited [Citation19,Citation20,Citation26]. Moreover, recent studies showing promise for proactive TDM of TNF inhibitors in IBD, have not been incorporated into the guidelines [Citation27–29]. In line with this, a few previous survey studies from US and UK showed high utilization of reactive TDM (87-97%) but not proactive TDM (36-54%) [Citation14,Citation15]. Interestingly, and likely reflecting the improved evidence level, proactive TDM was used by approximately two thirds of Scandinavian IBD physicians participating in our survey. Theoretical clinical case scenarios showed that physicians would generally continue the therapeutic regimen in case of therapeutic drug levels, most would intensify the regimen in case of sub-therapeutic drug concentration and de-escalate the regimen at supra-therapeutic drug levels. There were some disagreements on interventions preferably applied in case of ADAb detection – most would base their strategy depending on ADAb level – but with a clear trend to optimize either the biologic regimen or concomitant immunosuppressive treatments. The somewhat higher level of inconsistencies for proactive than reactive TDM interventions may reflect the lack of guidelines concerning proactive TDM, lack of evidence, or lack of knowledge on how to interpret test results. Of note, TDM was also frequently used at side-effects, at re-initiation of therapy, and when considering discontinuing biologic therapies; none of these indications are covered in existing guidelines.
Although TDM of biologics proved to be the standard of care for most participants, our study did reveal controversies. For example, a significantly lower proportion of responders from Denmark used TDM, and proactive TDM was exclusively used in the Capital Region of Copenhagen. In contrast, TDM of thiopurine therapies was commonly applied in Denmark. Thus, Danish participants reported reservations on the level of evidence favoring TDM. Furthermore, proactive TDM was more frequently used in Norway and by physicians handling a higher number of IBD patients per week. These observations suggest that differences in use may to some extent be explained by local traditions and preferences, and availability of competent laboratories. For example, Norway has recently conducted two high-quality proactive TDM studies and TDM analyses are easily available, centralized, and with low costs (∼20 EUR per assay) [Citation27,Citation32].
Three out of four participants noted important barriers that restricted their use of TDM. The most prominent barrier was a request for specific TDM guidelines. Thus, local proactive and reactive TDM guidelines were available only for about one-third, and only two-thirds reported awareness of international guidelines or expert consensus statements. Furthermore, almost half of participants felt a lack of knowledge on how to interpret TDM test results, and some also had reservations on the currently level of available evidence. Taken together, these observations originating from IBD clinicians clearly document an unmet need for updated, evidence-based, TDM guidelines and educational activities incorporating how to practically interpret TDM test results in the every-day clinical practice. Accordingly, we have here provided TDM recommendations for clinical practice below.
Long response time from the laboratory was also a commonly encountered barrier restricting TDM, except in Norway as mentioned above. Thus, the current study highlights the potential of point-of-care tests where serum drug levels and even ADAb can be assessed on-site at patient visits. These tests are now emerging and may serve to increase the use of TDM in clinical practice [Citation33,Citation34]. Costs were not a significant barrier in the Scandinavian countries as opposed to elsewhere, which is likely due to the low costs of TDM analyses and the Scandinavian health care systems where health expenses are covered via taxes [Citation13,Citation14].
This study also shows that TDM of thiopurines has now been fully adopted and widely implemented in clinical practice and routinely used both prior to initiation and reactively at treatment failure or side effects during ongoing therapy. This is consistent with guidelines [Citation19] and in line with a previous survey from Sweden [Citation35]. Albeit not recommended in guidelines, proactive measurements of thiopurine metabolites were, however, also routinely done once or twice a year. Thiopurines were generally used according to the conventional ‘step-up’ strategy, but a ‘top-down’ strategy was more common in Norway possibly due to biosimilars having been on the marked two years longer than elsewhere in Scandinavia. Main barriers were long response time from the laboratory, and only a minority requested TDM guidelines for thiopurines.
Major strengths of this study are the large number of participants from five different countries with a homogenous and representative study population of IBD specialists involved in biologic therapies. The survey was designed by clinicians having experience with TDM, was validated internally, and covered all currently available biologics and thiopurines. However, our results should be interpreted with caution as risk of selection bias is inherent in the study design due to responders not necessarily being representative, for example, responders may have a special interest in TDM. Furthermore, the number of participants from different countries was skewed, which can partly be ascribed to the different population sizes and thus differences in number of gastroenterologists in each country. Our results may not apply to other geographical regions due to e.g. differences in health care system, regional availability of TDM, and costs. Hence, investigation of TDM utilization and barriers in other regions and countries would be of interest in the future. However, being a developed region and with public health care systems, Scandinavia in theory provides an optimal setting for implementation of TDM.
In conclusion, TDM of biologic and thiopurine therapies have been broadly adopted and implemented in clinical practice in Scandinavia. However, physicians call for TDM guidelines detailing indications and interpretations of test results along with improved test response times. Importantly, in the view of precision medicine, TDM is important to predict outcomes in IBD patients, along with common and novel biomarkers Citation36.
Practical recommendations
In the following, we present practical recommendations on interpretation of therapeutic drug monitoring (TDM) test results during reactive (A) and proactive (B) TDM of biologics, recommended therapeutic thresholds for biologics (C), and TDM of thiopurines (D). These recommendations are based on current available evidence and existing TDM guidelines.
Reactive TDM algorithm during anti-TNF therapies
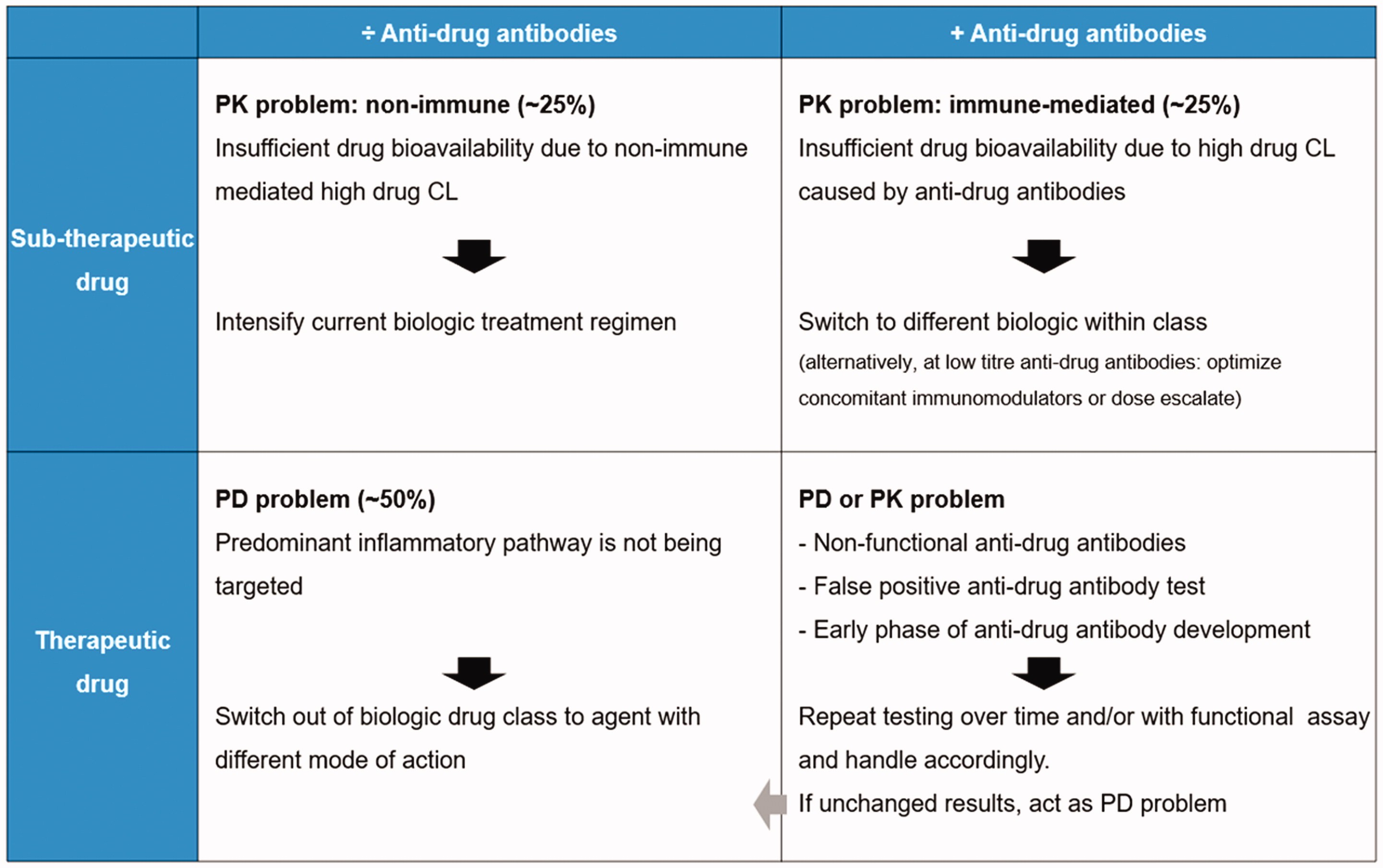
TDM recommended during induction and maintenance therapy and for all TNF-inhibitors [Citation7,Citation18–25,Citation37]. It is generally recommended to repeat testing over time. PK = pharmacokinetic PD = pharmacodynamic CL = clearance.
Proactive TDM algorithm during anti-TNF therapies
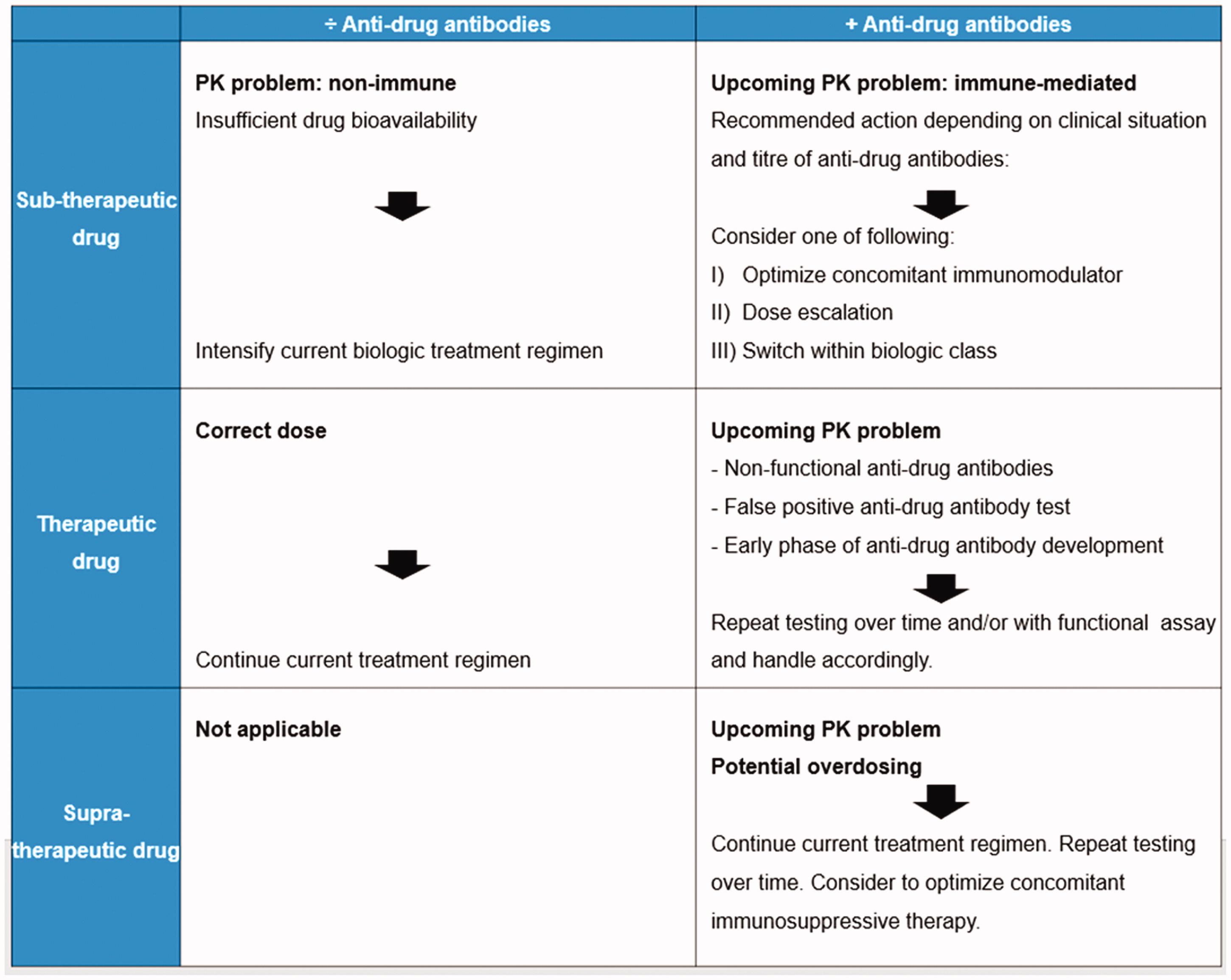
Proactive TDM following end of induction phase with dose optimization to achieve therapeutic drug level is recommended [Citation22,Citation25]. Routine proactive TDM recommended 1-2 times a year during maintenance and for all biologics [Citation18,Citation22]. It is generally recommended to repeat testing over time. PK = pharmacokinetic PD = pharmacodynamic.
Suggested therapeutic thresholds
TDM of thiopurines
TPMT genotype and phenotype assessment before treatment initiation should be used in all patients [Citation19].
Thiopurine metabolites (6-TGN and 6-MeMP) should be measured reactively at treatment failure or side-effects[Citation19].
Proactive measurement of thiopurine metabolites (6-TGN and 6-MeMP) is recommended at regular intervals for example once yearly, and more frequently in high-risk patients [Citation19,Citation40].
Authors contribution
Study concept and design: KHB + KKJ + CS + JJ; Acquisition of data: KHB + KKJ + CS; Analysis and interpretation of data: All authors; Drafting of the manuscript: KHB + KKJ + CS; Critical revision of the manuscript for important intellectual content: All authors; Final approval: All authors.
| Abbreviations | ||
| IBD | = | inflammatory bowel disease |
| TDM | = | therapeutic drug monitoring |
| ADAb | = | anti-drug antibodies |
| TNF | = | tumour necrosis factor |
| TPMT | = | thiopurine S-methyltransferase |
| 6-TGN | = | 6-thioguanine nucleotide |
| 6-MMP | = | 6-methylmercaptopurine |
Supplemental Tables
Download PDF (197.1 KB)Acknowledgments
The authors thank all IBD physicians in Scandinavia that participated in the survey. The authors thank Dr. Nils Bolstad at the Department of Medical Biochemistry at Oslo University Hospital Radiumhospitalet for advice regarding TDM analyses and assays.
Disclosure statement
KHB funding from Akershus University Hospital and speaker for Janssen-Cilag.
JJ reports personal fee as a speaker, consultant, or advisory board member for AbbVie, Boerhinger Ingelheim, BMS, Celltrion, Giliad, Hikma, Janssen Cilag, Novartis, Orion Pharma, Pfizer, Roche, Takeda and Sandoz.
JB Speaker-, educational- and advisory board consultancy fees and research grants from Abbvie, MSD, Pfizer, Takeda, BMS, Gilead and Janssen-Cilag.
PM speaker and consultancy fees from Abbvie, Orion Pharma, Pfizer, Sandoz, Takeda, Tillotts Pharma and advisory board member fees from BMS, Gilead and Janssen-Cilag.
ME none declared.
LGD none declared.
TS speaker, advisory board member and research grant from Janssen-Cilag, consultation and advisory board member for BMS, speaker for Celltrion, research grant from Takeda.
HH Lecture fee: Takeda, Janssen. Advisory board: Takeda, Janssen, Norgine, Abbvie, Pfizer, Fresenius kabi. Research grant: Ferring, Tillotts Pharma.
GLG speaker fees from AbbVie, Boehringer Ingelheim, Celltrion, Lilly, Orion Pharma, Novartis, Sandoz; advisory boards for AbbVie, Celltrion, Pfizer.
SWS none declared.
EL none declared.
KKJ speaker for Norgine, Roche and BMS.
CS speaker and advisory board member for MSD and Janssen-Cilag.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Additional information
Funding
References
- Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770.
- Torres J, Mehandru S, Colombel JF, et al. Crohn's disease. Lancet. 2017;389(10080):1741–1755.
- Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341–353.
- Colombel JF, D'Haens G, Lee WJ, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254–266.
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779–2789.
- Singh S, Murad MH, Fumery M, et al. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020;18(10):2179–2191.e6.
- Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63(6):919–927.
- Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017;15(10):1580–1588.e3.
- Papamichael K, Vajravelu RK, Vaughn BP, et al. Proactive infliximab monitoring following reactive testing is associated with better clinical outcomes than reactive testing alone in patients with inflammatory bowel disease. J Crohns Colitis. 2018;12(7):804–810.
- Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320–1329 e3.
- Steenholdt C, Bendtzen K, Brynskov J, et al. Optimizing treatment with TNF inhibitors in inflammatory bowel disease by monitoring drug levels and antidrug antibodies. Inflamm Bowel Dis. 2016;22(8):1999–2015.
- Papamichael K, Afif W, Drobne D, International Consortium for Therapeutic Drug Monitoring, et al. Therapeutic drug monitoring of biologics in inflammatory bowel disease: unmet needs and future perspectives. Lancet Gastroenterol Hepatol. 2022;7(2):171–185.
- Patel RN, Nigam GB, Jatale RG, et al. An Indian national survey of therapeutic drug monitoring with anti-tumor necrosis (TNF) medications in inflammatory bowel disease. Indian J Gastroenterol. 2020;39(2):176–185.
- Grossberg LB, Papamichael K, Feuerstein JD, et al. A survey study of gastroenterologists' attitudes and barriers Toward therapeutic drug monitoring of Anti-TNF therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(1):191–197.
- Nigam GB, Patel RN, Jatale RG, et al. UK national survey of gastroenterologists’ attitudes and barriers toward therapeutic drug monitoring of anti-TNF therapy in inflammatory bowel disease. Frontline Gastroenterology. 2020;158(6):S-246.
- Samaan MA, Arkir Z, Ahmad T, et al. Wide variation in the use and understanding of therapeutic drug monitoring for anti-TNF agents in inflammatory bowel disease: an inexact science? Expert Opin Biol Ther. 2018;18(12):1271–1279.
- Thomas PWA, Chin PKL, Barclay ML. A nationwide survey on therapeutic drug monitoring of anti-tumour necrosis factor agents for inflammatory bowel disease. Intern Med J. 2021;51(3):341–347.
- Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients With inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(9):1655–1668 e3.
- Feuerstein JD, Nguyen GC, Kupfer SS, American Gastroenterological Association Institute Clinical Guidelines Committee, et al. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153(3):827–834.
- Lamb CA, Kennedy NA, Raine T, IBD guidelines eDelphi consensus group, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106.
- Steinhart AH, Panaccione R, Targownik L, et al. Clinical practice guideline for the medical management of perianal fistulizing Crohn's disease: the Toronto consensus. Inflamm Bowel Dis. 2019;25(1):1–13.
- Cheifetz AS, Abreu MT, Afif W, et al. A comprehensive literature review and expert consensus statement on therapeutic drug monitoring of biologics in inflammatory bowel disease. Am J Gastroenterol. 2021;116(10):2014–2025.
- Vande Casteele N, Feagan BG, Wolf DC, et al. Therapeutic drug monitoring of tumor necrosis factor antagonists in crohn disease: a theoretical construct to apply pharmacokinetics and guidelines to clinical practice. Inflamm Bowel Dis. 2020;27(8):1346–1355.
- Mitrev N, Vande Casteele N, Seow CH, IBD Sydney Organisation and the Australian Inflammatory Bowel Diseases Consensus Working Group, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46(11-12):1037–1053.
- Sparrow MP, Papamichael K, Ward MG, et al. Therapeutic drug monitoring of biologics During induction to prevent primary Non-Response. J Crohns Colitis. 2020;14(4):542–556.
- Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22.
- Syversen SW, Jørgensen KK, Goll GL, et al. Effect of therapeutic drug monitoring vs standard therapy during maintenance infliximab therapy on disease control in patients with immune-mediated inflammatory diseases: a randomized clinical trial. Jama. 2021;326(23):2375–2384.
- Fernandes SR, Bernardo S, Simoes C, et al. Proactive infliximab drug monitoring Is superior to conventional management in inflammatory bowel disease. Inflamm Bowel Dis. 2020;26(2):263–270.
- Sánchez-Hernández JG, Rebollo N, Martin-Suarez A, et al. A 3-year prospective study of a multidisciplinary early proactive therapeutic drug monitoring programme of infliximab treatments in inflammatory bowel disease. Br J Clin Pharmacol. 2020;86(6):1165–1175.
- Coskun M, Steenholdt C, de Boer NK, et al. Pharmacology and optimization of thiopurines and methotrexate in inflammatory bowel disease. Clin Pharmacokinet. 2016;55(3):257–274.
- Kothari MM, Nguyen DL, Parekh NK. Strategies for overcoming anti-tumor necrosis factor drug antibodies in inflammatory bowel disease: case series and review of literature. World J Gastrointest Pharmacol Ther. 2017;8(3):155–161.
- Syversen SW, Goll GL, Jørgensen KK, et al. Effect of therapeutic drug monitoring vs standard therapy during infliximab induction on disease remission in patients with chronic immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. 2021;325(17):1744–1754.
- Van Stappen T, Bollen L, Vande Casteele N, et al. Rapid test for infliximab drug concentration allows immediate dose adaptation. Clin Transl Gastroenterol. 2016;7(12):e206.
- Verstockt B, Moors G, Bian S, et al. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn's disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther. 2018;48(7):731–739.
- Hindorf U, Andersson P. How are thiopurines used and monitored by swedish gastroenterologists when treating patients with inflammatory bowel disease? Scand J Gastroenterol. 2011;46(10):1215–1221.
- Cui G, Fan Q, Li Z, et al. Evaluation of anti-TNF therapeutic response in patients with inflammatory bowel disease: current and novel biomarkers. EBioMedicine. 2021;66:103329.
- Bendtzen K, Ainsworth M, Steenholdt C, et al. Individual medicine in inflammatory bowel disease: monitoring bioavailability, pharmacokinetics and immunogenicity of anti-tumour necrosis factor-alpha antibodies. Scand J Gastroenterol. 2009;44(7):774–781.
- Gibson DJ, Ward MG, Rentsch C, et al. Review article: determination of the therapeutic range for therapeutic drug monitoring of adalimumab and infliximab in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51(6):612–628.
- Shukla R, Ananthakrishnan A. Therapeutic drug monitoring of Non-Anti-Tumor necrosis factor biologics. Clin Gastroenterol Hepatol. 2021;19(6):1108–1110.
- Vande Casteele N, Herfarth H, Katz J, et al. American gastroenterological association institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017;153(3):835–857.e6.