Abstract
Arbuscular mycorrhizal (AM) fungi enhance plant uptake of available phosphorus (P) from soil through their extraradical hyphae. The mechanism underlying this P uptake enhanced by AM fungi is the increase in the surface area for absorption of available P. Little is known about utilization of unavailable P by AM fungi. We investigated whether extraradical hyphae of AM fungi release acid phosphatase (ACP). Sterilized Andosol was packed in pots that were separated into the mycorrhizal and hyphal compartments with a nylon net of 30-μm pore size. Seeds of Allium fistulosum L. were inoculated or uninoculated with the AM fungus Rhizophagus clarus (Nicolson & Schenck) Walker & Schüßler. Mullite ceramic tubes were buried in the soil of each compartment, and soil solution was collected. A. fistulosum L. and Linum usitatissimum L. inoculated with R. clarus were grown in sand culture and in vitro monoxenic culture, respectively. Uninoculated A. fistulosum L was grown in hydroponic culture to collect root exudate. The soil solution, hyphal extracts, root extract and root exudates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Shoot P concentration, shoot P content and shoot dry weight were higher in the inoculated treatment than in the uninoculated treatment. Activity staining of the gel revealed that ACP activity at 187 kDa was observed in the soil solution in the inoculation treatment, and in the hyphal extract collected from sand culture and in vitro monoxenic culture, but neither in the root exudate of non-mycorrhizal plant grown in the hydroponic culture nor in the root extracts irrespective of mycorrhizal status. Those results provide strong evidence that the corresponding activity in the soil solutions in soil culture is of R. clarus CK001 origin. These findings suggest that the fungus releases ACP from extraradical hyphae into the hyphosphere.
INTRODUCTION
Phosphate fertilizer is produced from phosphate rock. Despite the fact that phosphate fertilizer demand is projected to increase, global phosphate fertilizer production is estimated to peak around 2030, and phosphate rock may be depleted 50–100 years later (Cordell et al. Citation2009). Two strategies for tackling depletion of phosphate rock are considered. One is to develop plants that can recycle phosphorus (P) more efficiently within their bodies. The other is to develop plants that can take up phosphate more efficiently from the soil. The concentration of plant-available phosphate, i.e., free inorganic orthophosphate (Pi), in the soil is usually very low in most ecosystems, which frequently limits plant growth. On the other hand, large amounts of phosphate in the soil are present as plant-unavailable forms: sparingly soluble inorganic phosphate and organic phosphate. In particular, organic phosphate accounts for 20–80% of total phosphate in the soil (Dalai Citation1977) and, thus, conversion (hydrolysis) of organic phosphate into plant-available Pi is a key to sustainable food production.
Plants and fungi possess many genes encoding acid phosphatase (EC 3.1.3.2, ACP) in their genomes, e.g., 29 genes in the plant Arabidopsis thaliana (L.) Heynh. (Wang et al. Citation2014) and at least seven genes are expressed in Rhizophagus clarus (Nicolson & Schenck) Walker & Schüßler (e.g., Kikuchi and Ezawa 2012, unpublished data). Secretion of ACP into the rhizosphere/hyphosphere, however, has been demonstrated only in limited ranges of plants (e.g., Tarafdar and Claassen Citation1988; Tadano and Sakai Citation1991) and fungi (e.g., Van Aarle and Plassard Citation2010; Crowther et al. Citation2011). In plants, secreted ACP hydrolyzes organic phosphate, increases the Pi pool in the rhizosphere and thus enhances Pi uptake (Wasaki et al. Citation2009; Maruyama et al. Citation2012; Robinson et al. Citation2012).
Arbuscular mycorrhizal (AM) fungi associate with 80% of land plants and enhance Pi uptake of plants from the soil through their extraradical hyphae (Smith and Read Citation2008). It has been considered that the increase in surface area for Pi uptake is the main mechanism underlying the enhanced Pi uptake in the associations. In addition to this mechanism, the involvement of AM fungal ACP in the enhanced Pi uptake has also been proposed. Tarafdar and Marschner (Citation1994) employed a compartment culture system in which the mycorrhizal (i.e., roots + hyphae) and hyphal compartments were separated by a 30-μm nylon mesh, and observed higher ACP activity in the hyphal compartment in the presence of an AM fungus Glomus mosseae Nicolson & Gerd., suggesting that the fungus secreted ACP from extraradical hyphae. Koide and Kabir (Citation2000) demonstrated hydrolysis of organic phosphate in the hyphal compartment of the in vitro two-compartment monoxenic culture of G. intraradices Schenck & Smith with a concurrent increase in root P content, suggesting that plants can access organic phosphate via fungal ACP secretion. Joner and Johansen (Citation2000) found that fungal ACP was mostly associated with the cell wall and released ACP was undetectable, results also reproduced by Olsson et al. (Citation2002).
In plants, not only cell wall-bound ACP but also released ACP play a significant role in organic P hydrolysis in the rhizosphere. In Arabidopsis thaliana, two purple acid phosphatases encoded by AtPAP12 and AtPAP26 are released from the roots (Tran et al. Citation2010), and the corresponding knockout mutants showed poorer growth than the wild type in the presence of organic P (fructose-6-phosphate) as the sole P source in the medium, suggesting that ACP released from the roots greatly contributes to plant growth (Wang et al. Citation2014). Although these observations led us to hypothesize that AM fungi also release ACP into the soil solution, a technical breakthrough was necessary for the collection and detection of released ACP.
The exudation of organic acid by extraradical hyphae of an AM fungus has been successfully demonstrated in the soil solution collected using mullite ceramic tubes in a two-compartment culture system (Tawaraya et al. Citation2006). In the present study, this collection technique was employed for the qualitative assessments of AM fungal ACP exuded in the soil solution, to test the hypothesis that AM fungi exude soluble ACP from extraradical hyphae.
MATERIALS AND METHODS
Two-compartment culture for collection of soil solution
Andosol was collected from a native pasture in Haguromachi, Yamagata prefecture, Japan. The soil was air-dried in a glass house, sieved on a 2-mm sieve and steam-sterilized twice at 80°C for 45 min. Chemical properties of the soil were as follows: pH (H2O), 4.78; organic carbon, 10.9%; total nitrogen, 0.88%; Truog-P, 5.02 mg P kg soil−1; cation exchange capacity, 43.2 cmol (+) kg soil−1. The soil was fertilized with ammonium sulfate, potassium sulfate and superphosphate at rates of 1.00 g nitrogen (N), 0.83 g potassium (K) and 0.15 g P kg−1 soil, respectively, and pH was adjusted to pH 5.1 with calcium carbonate at a rate of 4.19 g kg−1 soil. The AM fungus Rhizophagus clarus (T.H. Nicolson & N.C. Schenck) C. Walker & A. Schüßler strain CK001 was propagated with Welsh onion (Allium fistulosum L. cv. Motokura), sorghum (Sorghum bicolor (L.) Moench. cv. New sorghum 2 gou), and white clover (Trifolium repens L. cv. California ladino) for 3 months, and the soil that included spores, extraradical hyphae and the roots was used as inoculum. The two-compartment pots were prepared as described by Tawaraya et al. (Citation2006). A nylon bag (top 140 mm, bottom 80 mm, height 90 mm) was prepared with a 30-μm nylon net (NY30HD, Sefar Inc., Heiden, Switzerland), though which only fungal hyphae are able to pass, but not plant roots, filled with soil-inoculum layers as follows: 20 g of the sterilized soil in the bottom, 10 g of the inoculum as the second layer, 20 g of the sterilized soil as the third layer and 10 g of the inoculum as the fourth layer, and then covered with 40 g of the sterilized soil (mycorrhizal compartment). For the uninoculated treatment, no inoculum layers were incorporated in the compartment. The nylon bag was placed at the center of a 500-mL plastic pot (11.5 × 9.5 cm), and the remaining space was filled with 300 g of the sterilized soil (hyphal compartment). Each six mullite ceramic tubes (50 × 2.5 mm, 1DH-1525, Sakaguchi E.H. VOC Corp., Tokyo, Japan) connected to Teflon tubes (150 × 1.5 mm) were embedded in the mycorrhizal and hyphal compartments at intervals of 2 and 3 cm in a circular pattern, respectively (Tawaraya et al. Citation2006). There were five replications of each treatment. Twelve seeds of Welsh onion were sown in the mycorrhizal compartment at 1 cm depth, irrigated with deionized water to maintain the water potential at −0.03 Mpa and grown in a 16-h photoperiod (150 μmol m−2 s−1) at 27°C/25°C (light/dark) in a growth chamber. The pots were irrigated with deionized water every other day. Each pot was covered until germination, and plants were thinned to six per pot. A plastic syringe (5-mL plastic syringe, TOP Co., Ltd., Tokyo, Japan) was connected to a Teflon tube, and 1 mL of soil solution was collected into the syringe by pulling the piston several times. The solution was collected from all tubes 40, 45, 50 and 55 d after sowing, stored at −30°C, and pooled before concentration. Shoots and roots were harvested 55 d after sowing. Shoots were weighted after drying at 70°C for 72 h and were used for plant P assessment. Roots were washed with tap water, wiped with a paper towel, weighed and used for enzyme preparation and assessment of mycorrhizal colonization.
Sand culture for preparation of extraradical hyphae
Welsh onion inoculated with R. clarus CK001 was grown on sterilized (121°C, 45 min) sea sand in a glass house. A nutrient solution [40 mg N L–1 (ammonium nitrate, NH4NO3), 20 mg N L–1 (sodium nitrate, NaNO3), 60 mg K L–1 (potassium sulfate, K2SO4), 80 mg Ca L–1 (calcium chloride, CaCl2), 40 mg Mg L–1 (magnesium sulfate, MgSO4), 2 mg Fe L–1 (iron(II) sulfate, FeSO4), 1 mg Mn L–1 (manganese sulfate, MnSO4), 0.01 mg Cu L–1 (copper sulfate, CuSO4), 0.005 Mo mg L–1 [ammonium molybdate, (NH4)6Mo7O24], 0.4 mg B L–1 (boric acid, H3BO3), 0.2 mg Zn L–1 (zinc chloride, ZnCl2), and 1 mg P L–1 (sodium dihydrogen phosphate, NaH2PO4)] (Wagatsuma et al. Citation1988) was applied to plants every other day. Extraradical hyphae were collected 45 d after sowing by wet sieving, wiped with a paper towel, weighed, stored at −30°C and used for enzyme extraction.
In vitro monoxenic culture for preparation of extraradical hyphae
Agrobacterium rhizogenes-transformed roots (hairy roots) of flax (Linum usitatissimum L.) purchased from Glomeromycota in vitro collection (http://www.mycorrhiza.be/ginco-bel/) were inoculated with surface-sterilized R. clarus CK001 spores and grown on modified Strullu–Romand (MSR) medium (Declerck et al. Citation1998) for 60 d in 9-cm Petri dishes according to Chabot et al. (Citation1992). After removing the roots with tweezers, the culture medium of 30 Petri dishes was transferred to 1 L of 10 mM sodium citrate (pH 6.0) to solubilize the Gellan Gum medium (Doner and Becard Citation1991). Extraradical hyphae were collected on a 30-μm nylon net, washed with sterilized water, wiped with a paper towel, weighed and used for enzyme extraction.
Hydroponic culture for collection of root exudate
Seeds of Welsh onion were sown on moist vermiculite spread. One week after sowing, 30 seedlings were carefully lifted out of the vermiculite, fixed with a sponge in a paper cup filled with 200 mL of the nutrient solution without P, and grown in the growth chamber (light 16 h/dark 8 h, 150 μmol m−2 s−1, 27°C light/25°C dark). The pH of the nutrient solution was adjusted daily to 5.0 ± 0.05 with 0.5 M sulfuric acid (H2SO4) and 0.5 M sodium hydroxide (NaOH). The nutrient solution was aerated with an air pump and replaced weekly. Thirteen days after transplanting, the seedlings were transferred to 50 mL of sterile deionized water and incubated for 12 h under the same conditions to collect root exudate, and then the solution was stored at –30°C and used for qualitative analysis.
Enzyme preparation
Soil solution and root exudates were first concentrated over 200-fold using Amicon® Ultra-15 centrifugal filter units (Millipore, MA, USA), further to 1800- and 500-fold, respectively, using Microcon® YM-30 centrifugal filter units (Millipore Corporation, MA, USA), and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Roots and extraradical hyphae were ground using mortar and pestle at 0°C with an equal volume (weight/volume) of 100 mM borate buffer (pH 8.8) with 10 μL mL−1 protease inhibitor (Plant ProteaseArrest™, Takara Bio Inc., Shiga, Japan). The resulting slurry was transferred to a 1.5-mL tube and centrifuged at 15,000 × g for 30 min at 4°C. The supernatant of root extract was transferred to a new tube and subjected to SDS-PAGE. The supernatant of hyphal extract was concentrated 24-fold using Microcon® YM-30 centrifugal filter units and subjected to SDS-PAGE.
Qualitative analysis of acid phosphatase
SDS-PAGE was performed as described by Ezawa and Yoshida (Citation1994). Ten microliters each of the soil solution, root exudate, hyphal extract and root extract were loaded on a 10% SDS-polyacrylamide gel without denaturing and electrophoresed. The gel was gently shaken in washing buffer [50 mL of 100 mM acetate/sodium buffer (pH 5.0) containing 0.5 mL of Triton-X] for 30 min, and ACP activity on the gel was visualized by the azo dye method (Scandalios Citation1969). The molecular weight of protein was estimated based on mobility relative to those of the protein standards (Takara Bio Inc. Shiga, Japan) on the gel.
Assessment of plant P and mycorrhizal colonization
Ground shoots were digested in a nitric acid (HNO3)-perchloric acid (HClO4)-sulfuric acid (H2SO4) (5:2:1) solution. The P content in the digested solution was determined colorimetrically by vanadomolybdate-yellow assay (Olsen and Sommers Citation1982).
Percent AM colonization was determined using the gridline intersect method (Giovannetti and Mosse Citation1980) after root staining with 500 mg L−1 aniline blue solution (Tawaraya et al. Citation1998). One hundred root segments from each replicate bag were observed under a microscope (magnification, 100×).
Analysis of variance (ANOVA) with Tukey-honestly significant difference (HSD) test (p < 0.05) was performed with Kaleida Graph 4.0 j (HULINKS Inc., Tokyo, Japan).
RESULTS
Qualitative characterization of acid phosphatase released from mycorrhiza
AM colonization was observed in the inoculated treatment 55 d after sowing, but not in the uninoculated treatment. Shoot P concentration, P content and dry weight were higher in the inoculated treatment than in the uninoculated treatment ().
Table 1 Arbuscular mycorrhizal (AM) colonization, shoot P concentration, shoot P content, and shoot dry weight of Welsh onion (Allium fistulosum L.) without (-) or with (+) AM fungi (AMF) Rhizophagus clarus (Nicolson & Schenck) Walker & Schüßler strain CK001 inoculation 55 days after sowing. S.E. indicates standard error. Values followed by a different letter are significantly different (P < 0.05)
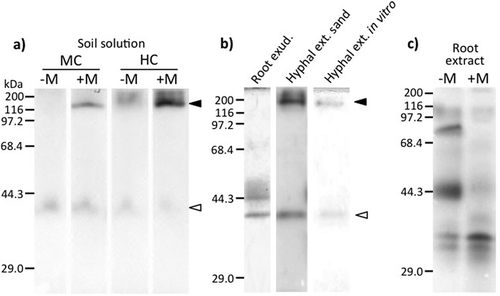
Activity staining of the gel revealed that ACP activity at 187 kDa was observed exclusively in the presence of the fungus in the solutions obtained from the mycorrhizal and hyphal compartments in the soil culture (). This activity was also detected in the extracts of extraradical hyphae grown both in the sand culture and the in vitro monoxenic culture, but neither in the exudate of non-mycorrhizal roots grown in the hydroponic culture nor in the root extracts irrespective of mycorrhizal status ( and ). ACP activity at 41 kDa was observed in all samples irrespective of mycorrhizal status, except for the root extracts.
Figure 1 SDS-PAGE analysis of acid phosphatase (ACP) activity of 1800-fold concentrated soil solution collected from the mycorrhizal compartment (MC) and hyphal compartment (HC) of Welsh onion (Allium fistulosum L.) inoculated with (+M) or without (–M) arbuscular mycorrhizal (AM) fungus R. clarus CK001 (a), 24-fold concentrated extraradical hyphal extract from Rhizophagus clarus (Nicolson & Schenck) Walker & Schüßler strain CK001 collected from sand culture (Hyphal ext. sand) or in vitro monoxenic culture (Hyphal ext. in vitro), 500-fold concentrated root exudates (Root exud.) from Welsh onion grown on P-free nutrient solution (b), and root extract from Welsh onion inoculated with (+M) or without (–M) AM fungus R. clarus CK001 (c). Black and white arrows indicate fungal ACP activity.
DISCUSSION
Release of ACP from extraradical hyphae of AM fungus R. clarus
The detection of the ACP activity of 187 kDa in the in vitro culture provides strong evidence that the corresponding activity in the soil solutions in sand culture is of R. clarus CK001 origin, suggesting that the fungus releases ACP from extraradical hyphae into the hyphosphere. This finding is further supported by the absence of the activity in the root exudate and extract. The detection of the ACP activity in the in vitro culture also excludes the possibility that the activity was of other microorganisms present in the rhizosphere/hyphosphere.
We demonstrated for the first time using mullite ceramic tubes in conjunction with SDS-PAGE that AM fungi release ACP from the extraradical hyphae to the hyphosphere. A large part of extracellular ACP has been considered to be bound to the cell wall in AM fungi, because released ACP fraction collected from the culture medium i.e. polysaccharide gel in the in vitro culture (Koide and Kabir, Citation2000; Olsson et al., Citation2002) or that collected by 1 h incubation of hyphae in a buffer (Joner and Johansen, Citation2000) showed little activity. The successful detection of released ACP activity in the present study was likely to be achieved by the concentration of the activity up to three orders of magnitude by ultrafiltration.
Although fungi have several ACP orthologues in their genome (for example, at least seven genes are expressed in R. clarus, e.g., Kikuchi and Ezawa 2012, unpublished data), molecular characterization of ACP of AM fungi has been rarely reported. Additionally, mobility on the PAGE analysis is modified by glycosylation (Weber and Pitt Citation1997). Thus, estimation of the gene is impossible. The identification of the ACP of 187 kDa is necessary using an immunological approach and the genetic information of known ACPs.
Although ACP of 41 kDa was detected in all soil solutions irrespective of mycorrhizal status, it seems unlikely that the activity is solely of the host plant Welsh onion. This is because the activity was detected not only in the hyphal extract in the sand culture but also that in the in vitro culture, in which different host plants, onion for the sand culture and flax for the in vitro culture, were used to grow the fungus, raising two possibilities: the two plants exude ACPs of similar molecular weight that migrate at similar rates on the gel, or onion and the fungus possess ACPs of similar molecular weight, which were in either case indistinguishable in electrophoresis. The ACP of 41 kDa was detected in root exudates, but not root extracts. The reason for the incompatible results might be due to different mobility caused by the formation of a dimer (Ozawa et al. Citation1995), or glycosylation (Weber and Pitt Citation1997). Further biochemical characterization and identification of genes encoding these ACPs would be necessary to test the possibilities.
Our results indicate that the AM fungus R. clarus CK001 releases ACP from its extraradical hyphae. The increase in surface area for Pi uptake with extension of extraradical hyphae beyond the P-depletion zone of rhizosphere soil is the mechanism of enhanced P acquisition of mycorrhizal plants. The present study can propose a new mechanism that extraradical hyphae of AMF hydrolyse soil organic P and enhance P acquisition. The hydrolysis of soil organic P with released ACP and the uptake of the resultant Pi by extraradical hyphae remain to be clarified.
ACKNOWLEDGMENTS
This study was supported by a Grant-in-Aid for Scientific Research (No. 23580086) from the Japan Society for the Promotion of Science (JSPS).
REFERENCES
- Chabot S, Becard G, Piche Y 1992: Life cycle of Glomus intraradix in root organ culture. Mycologia, 84, 315–321. doi:10.2307/3760183
- Cordell D, Drangert J-O, White S 2009: The story of phosphorus: global food security and food for thought. Glob. Environ. Chang., 19, 292–305. doi:10.1016/j.gloenvcha.2008.10.009
- Crowther TW, Jones TH, Boddy L, Baldrian P 2011: Invertebrate grazing determines enzyme production by basidiomycete fungi. Soil Biol. Biochem., 43(10), 2060–2068. doi:10.1016/j.soilbio.2011.06.003
- Dalai RC 1977: Soil organic phosphorus. Adv. Agron., 29, 83–117. doi:10.1016/S0065-2113(08)60216-3
- Declerck S, Strullu DG, Plenchette C 1998: Monoxenic culture of the intraradical forms of Glomus sp. isolated from a tropical ecosystem: a proposed methodology for germplasm collection. Mycologia, 90, 579–585. doi:10.2307/3761216
- Doner LW, Becard G 1991: Solubilization of gellan gels by chelation of cations. Biotechnol. Tech., 5, 25–28. doi:10.1007/BF00152749
- Ezawa T, Yoshida T 1994: Characterization of phosphatase in marigold roots infected with vesicular-arbuscular mycorrhizal fungi. Soil Sci. Plant Nutr., 40, 255–264. doi:10.1080/00380768.1994.10413299
- Giovannetti M, Mosse B 1980: An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol., 84, 489–500. doi:10.1111/j.1469-8137.1980.tb04556.x
- Joner EJ, Johansen A 2000: Phosphatase activity of external hyphae of two arbuscular mycorrhizal fungi. Mycol. Res., 104, 81–86. doi:10.1017/S0953756299001240
- Koide RT, Kabir Z 2000: Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol., 148, 511–517. doi:10.1046/j.1469-8137.2000.00776.x
- Maruyama H, Yamamura T, Kaneko Y, Matsui H, Watanabe T, Shinano T, Osaki M, Wasaki J 2012: Effect of exogenous phosphatase and phytase activities on organic phosphate mobilization in soils with different phosphate adsorption capacities. Soil Sci. Plant Nutr., 58, 41–51. doi:10.1080/00380768.2012.656298
- Olsen SR, Sommers LE 1982: Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, Ed. Page AL, pp. 403–430. American Society of Agronomy, Madison, WI.
- Olsson PA, Van Aarle IM, Allaway WG, Ashford AE, Rouhier H 2002: Phosphorus effects on metabolic processes in monoxenic arbuscular mycorrhiza cultures. Plant Physiol., 130, 1162–1171. doi:10.1104/pp.009639
- Ozawa K, Osaki M, Matsui H, Honma M, Tadano T 1995: Purification and properties of acid phosphatase secreted from lupin roots under phosphorus-deficiency conditions. Soil Sci. Plant Nutr., 41, 461–469. doi:10.1080/00380768.1995.10419608
- Robinson WD, Park J, Tran HT, Del Vecchio HA, Ying S, Zins JL, Patel K, McKnight TD, Plaxton WC 2012: The secreted purple acid phosphatase isozymes AtPAP12 and AtPAP26 play a pivotal role in extracellular phosphate-scavenging by Arabidopsis thaliana. J. Exp. Bot., 63, 6531–6542. doi:10.1093/jxb/ers309
- Scandalios JG 1969: Genetic control of multiple molecular forms of enzymes in plants: a review. Biochem. Genet., 3, 37–79. doi:10.1007/BF00485973
- Smith SE, Read DJ 2008: Mycorrhizal Symbiosis, 3rd ed. Academic Press, London, UK.
- Tadano T, Sakai H 1991: Secretion of acid phosphatase by the roots of several crop species under phosphorus-deficient conditions Secretion of Acid Phosphatase by the Roots of Several Crop Species under Phosphorus-Deficient Conditions. Soil Soil Sci. Plant Nutr. 37, 129–140.
- Tarafdar JC, Claassen N 1988: Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biol. Fertil. Soils, 5, 308–312. doi:10.1007/BF00262137
- Tarafdar JC, Marschner H 1994: Phosphatase activity in the rhizosphere and Hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus. Soil Biol. Biochem., 26, 387–395. doi:10.1016/0038-0717(94)90288-7
- Tawaraya K, Hashimoto K, Wagatsuma T 1998: Effect of root exudate fractions from P-deficient and P-sufficient onion plants on root colonisation by the arbuscular mycorrhizal fungus Gigaspora margarita. Mycorrhiza, 8, 67–70. doi:10.1007/s005720050214
- Tawaraya K, Naito M, Wagatsuma T 2006: Solubilization of insoluble inorganic phosphate by hyphal exudates of arbuscular mycorrhizal fungi. J. Plant Nutr., 29, 657–665. doi:10.1080/01904160600564428
- Tran HT, Qian WQ, Hurley BA, She Y-M, Wang DW, Plaxton WC 2010: Biochemical and molecular characterization of AtPAP12 and AtPAP26: the predominant purple acid phosphatase isozymes secreted by phosphate-starved Arabidopsis thaliana. Plant Cell Environ., 33, 1789–1803. doi:10.1111/j.1365-3040.2010.02184.x
- Van Aarle IM, Plassard C 2010: Spatial distribution of phosphatase activity associated with ectomycorrhizal plants is related to soil type. Soil Biol. Biochem., 42, 324–330. doi:10.1016/j.soilbio.2009.11.011
- Wagatsuma T, Kawashima T, Tawaraya K 1988: Comparative stainability of plant root cells with basic dye (methylene blue) in association with aluminum tolerance. Commun. Soil Sci. Plant Anal., 19, 1207–1215. doi:10.1080/00103628809368006
- Wang LS, Lu S, Zhang Y, Li Z, Du XQ, Liu D 2014: Comparative genetic analysis of Arabidopsis purple acid phosphatases AtPAP10, AtPAP12, and AtPAP26 provides new insights into their roles in plant adaptation to phosphate deprivation. J. Integr. Plant Biol., 56, 299–314. doi:10.1111/jipb.12184
- Wasaki J, Maruyama H, Tanaka M, Yamamura T, Dateki H, Shinano T, Ito S, Osaki M 2009: Overexpression of the LASAP2 gene for secretory acid phosphatase in white lupin improves the phosphorus uptake and growth of tobacco plants. Soil Sci. Plant Nutr., 55, 107–113. doi:10.1111/j.1747-0765.2008.00329.x
- Weber RWS, Pitt D 1997: Purification, characterization and exit routes of two acid phosphatases secreted by Botrytis cinerea. Mycol. Res., 101, 1431–1439. doi:10.1017/S0953756297004139
