ABSTRACT
White lupin (Lupinus albus L.) plants are tolerant plants under phosphorus (P)deficiency. They form unique morphological roots, so-called cluster roots (CRs) under P deficiency. CRs contribute to P absorption by the expansion of the root surface area and P mobilization activities. Previous research has implied the involvement of several hormonal functions in CR formation. Ethylene is a key regulator responsible for the modification of root architecture and P acquisition in response to low P in plants. However, understanding the effect of ethylene on CR morphogenesis is not enough. Here, the focus was on the effects of ethylene on CR morphology and gene expression for P acquisition. First, a reanalysis of public RNA-Seq data indicated that the gene expression for ethylene synthesis was induced during CR maturation. In turn, the 10-days application of an ethylene synthesis inhibitor, CoCl2, and an ethylene precursor, ACC, to CR formed in hydroponic culture without P was performed. CR morphology, transcript levels of the genes related to P acquisition, and citrate concentration in roots were determined. The results indicated that the elongation of rootlets in CR was promoted in a Co2+ concentration-dependent manner, suggesting that ethylene is responsible for the arrest of rootlet elongation. mRNA accumulation for acid phosphatases, phosphate transporters, citrate synthases, and a putative citrate transporter increased in ACC-treated immature CR, suggesting that ethylene induces the transcription of genes for P acquisition. Additionally, the trend of citrate concentration in roots among treatments was similar to that in the expression of citrate synthases, supporting that ethylene accumulation promotes citrate synthesis. The roles of the arrest of rootlet elongation and regulation of gene expressions for P acquisition are considered independent functions of ethylene. It was concluded that ethylene works as a possible regulator for the rootlet elongation and transcription of genes for P acquisition in CRs, although further studies are required to elucidate the molecular mechanisms of the arrest of rootlet elongation and transcriptional regulation.
1. Introduction
Phosphorus (P) is one of the essential elements for plants and is required for many essential metabolic activities, such as nucleic acid synthesis, photosynthesis, and phospholipid synthesis (Vance, Uhde-Stone, and Allan Citation2003). Plant growth is extremely limited under low P conditions, and therefore, P fertilizers are vital for stable food production. Recently, however, we are facing the deprivation of resources for P fertilizers in the near future: it is estimated that the manufacture of P resources will be reduced from 2033 on (Cordell, Drangert, and White Citation2008). Therefore, it is urgent to develop a technology enabling stable yields of crops with fewer P fertilizers.
Soluble inorganic phosphate is the available form for plants; however, a large part of P in soils exists as unavailable forms (Schachtman, Reid, and Ayling Citation1998): phosphate is strongly bound by metal cations, such as Fe, Al, and Ca, and organic ligands in soils (Hinsinger Citation2001). Plants have developed several strategies to overcome low P conditions: one of the strategies is the enhancement of P absorption in roots (Vance, Uhde-Stone, and Allan Citation2003). In this strategy, plant roots secrete acid phosphatase, which hydrolyzes P-containing organic compounds, and organic acids, which also mobilize P contained in inorganic compounds, into the rhizosphere (Abel, Ticconi, and Delatorre Citation2002). The morphological response of roots also contributes to the improved P absorption under low P conditions (Peret et al. Citation2011). Plants under low P conditions suppress primary root elongation and alternatively increase lateral roots and root hairs, which increase the surface area of roots and promote P absorption (Bichara, Mazzafera, and de Andrade Citation2021; Peret et al. Citation2011). Some plant species grown in low P soils can form a unique root structure like bottle-brush, so-called ‘cluster roots (CRs),’ in response to low P conditions (Vance, Uhde-Stone, and Allan Citation2003). The lateral rootlets of CRs are restricted in length and densely spaced, which increases root surface in a restricted space (Skene Citation2000). Moreover, the secretions of acid phosphatase and organic acids are highly activated in CRs, enhancing P absorption (Lambers et al. Citation2006; Neumann and Martinoia Citation2002). CRs are thought to be involved in the low P tolerance (Lambers, Martinoia, and Renton Citation2015). Eight dicotyledonous families were recognized to form CRs in previous studies (Lambers et al. Citation2006). Among them, the crop species white lupin (Lupinus albus L.) of the Fabaceae has been well studied for the physiological functions and development mechanism of CRs. In white lupin, gene expression and physiological activities involved in P absorption are elevated in the CRs during the maturation of CRs (Shane et al. Citation2004; Tang et al. Citation2013). It was described that white lupin with CRs obtained five times more P per unit root length than soybean, which has no ability to form CRs (Watt and Evans Citation2003). Hufnagel et al. (Citation2020) presented a high-quality genome sequence of a modern accession of white lupin (2n = 50, 451 Mb) as a model species of CR-forming plants, allowing us to perform in-depth analysis.
A transcriptome analysis using RNA-Seq for the different developmental stages of CRs showed that several auxin-responsive genes (IAA4/5, IAA8, YUCCA, AUX, and PIN1) were highly expressed in 2–3 cm behind the root tips of lateral roots where CR primordia begins to form (Wang et al. Citation2014). A reporter assay for auxin accumulation showed that auxin was accumulated in the CR primordia, confirming that auxin accumulation is involved in the CR development (Gallardo et al. Citation2019). In the zone accumulating auxin, a periclinal division in the pericycle close to a protoxylem pole occurs, and dome-shaped primordium outgrowing from pericycle cells breaches endodermis, the cortex, and epidermis, resulting in the emergence of the cluster rootlets. This process is similar to the lateral root development in Arabidopsis (Du and Scheres Citation2018).
It is implied that the other hormones, including ethylene, are also involved in CR development (Wang et al. Citation2014, Citation2015). Ethylene is known as a key regulator responsible for the modification of root architecture in response to low P in plants: ethylene produced in response to low P suppresses primary root elongation and induces root hair development (Lei et al. Citation2011; Liu et al. Citation2017; Song et al. Citation2016). Ethylene is also produced in roots of white lupin under low P conditions (Gilbert et al. Citation2000), suggesting that ethylene is involved in the morphological response of roots to low P conditions in white lupin. Additionally, ethylene works as a positive regulator for expressing a subset of P starvation-induced genes, such as phosphate transporters, a non-coding transcript, an acid phosphatase, and a ribonuclease in Arabidopsis (Song and Liu Citation2015). This study aimed to reveal the role of ethylene in CR development and the high ability to P acquisition in white lupin. We investigated the expression patterns of genes involved in ethylene biosynthesis in CRs at different developmental stages and normal roots by analyzing public RNA-Seq data (by Secco et al. Citation2014) and reverse transcription-quantitative PCR. The effects of treatments with the ethylene biosynthesis inhibitor and ethylene precursor on CR morphology as well as P acquisition were also investigated.
2. Materials and methods
2.1. RNA-Seq analysis
The reference genome sequence of white lupin (version 1.0; Hufnagel et al. Citation2020), the gene annotation file, and the RNA-Seq data, which were obtained from the cluster roots and normal roots on Illumina HiSeq platform (Secco et al. Citation2014), were downloaded from the White LupinGenome website (https://www.whitelupin.fr). The RNA-Seq reads were mapped to the white lupin reference genome using TopHat2 (version 2.0.11; https://ccb.jhu.edu/software/tophat/index.shtml) after cleaning with Trimmomatic (version 0.32; http://www.usadellab.org/cms/index.php?page=trimmomatic). Gene expression level was quantified for every gene using HT-Seq (version 0.6.1p1; https://htseq.readthedocs.io/en/master/) and expressed as count per million (CPM). Genes showing low signal (CPM < 0.4) were removed according to a previous study (Nishida et al. Citation2017). Gene expression data were normalized across the samples, and pairwise comparison analyses between the groups were conducted using edgeR (version 4.0.3; https://www.r-project.org/) to detect differentially expressed genes (DEGs) with q-value < 0.05 and fold change (FC) ≧ 2.
2.2. Plant material and growth conditions
Seeds of white lupin (L. albus L. cv. Kievskij mutant) were surface sterilized with 70% (v/v) ethanol for 3 min and 10% (v/v) sodium hypochlorite solution for 3 min, rinsed thoroughly in running tap water for 2 days, and germinated in vermiculite for 5 days. Six seedlings were transferred onto a float and hydroponically cultured in a container filled with 4 L nutrient solution (2.1 mM NH4NO3, 0.77 mM K2SO4, 1.2 mM CaCl2, 0.82 mM MgSO4, 36 µM Fe-ethylenediaminetetraacetic acid, 9.1 µM MnSO4, 46 µM H3BO3, 3.1 µM ZnSO4, 0.16 µM CuSO4, 0.052 µM (NH4)6-Mo7O24, with or without 64 µM NaH2PO4 for P-sufficient condition or P-deficient condition, respectively, at an initial pH of 5.6–5.8) with continuous aeration. The containers were protected on the outside by black tape to shield root environment from light. The nutrient solution was replaced every 2 days during preculture and every day during treatments. Plants were grown in a growth chamber (LH-241S, NKsystem, Osaka, Japan) under 16 hours light (320 µmol/m2/s) at 25°C and 8 hours dark at 22°C.
2.3. Effect of exogenous ethylene synthesis inhibitor and ethylene precursor on the rootlet elongation and function of cluster roots
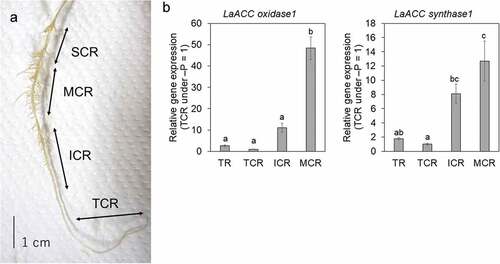
The 15-day-old seedlings were exposed to 0, 10, 20, or 40 µM CoCl2, which is the ethylene synthesis inhibitor (Lau and Yang Citation1976), in the P-deficient condition for 9 days in hydroponic culture. The experiment was performed with three biological replicates for each treatment. The CR-developing roots in the P-deficient condition were divided into four sections according to Secco et al. (Citation2014): the tip of CR, including root meristem (TCR) corresponding to the region above 3 cm from the root tip, immature CR (ICR), mature CR (MCR) with the fully elongated rootlets, and senescent CR (SCR) showing a dark brown colorization due to lignification (). The length of the top five longest rootlets in each MCR was measured using the image data, and the average value was calculated for each replicate.
Figure 1. Cluster root (CR) developmental stages in white lupin and relative gene expression for LaACC oxidase1 and LaACC synthase1 in 3 developmental stages.
The 18-day-old seedlings were exposed to 30 µM CoCl2 or 0.1 µM 1-aminocyclopropane-1-carboxylic acid (ACC), which is the precursor of ethylene enhancing ethylene accumulation in plants (Locke, Bryce, and Morris Citation2000), for 9 days in the P-deficient conditions. Plants grown in the P-deficient or P-sufficient conditions without exposure (CoCl2 and ACC) were also prepared. The experiment was conducted with five biological replicates for each treatment. The numbers of ICR, MCR, and SCR were determined, and each root section (TCR, ICR, MCR, and SCR of plants in the P-deficient condition, and TR: the tip of roots of plants in the P-sufficient condition) was immediately frozen in liquid nitrogen and stored at −80°C until analysis. The fresh weights of shoots and roots were determined before and after sampling of the root sections. The rest of the samples were oven-dried at 70°C for more than 3 days, and the dry weight was determined. The root dry weights before sampling the root sections were estimated with the dry and fresh weights determined above. The length of the top five longest rootlets in each MCR was measured using the image data, and the average value was calculated for each replicate. The frozen samples were used for quantitative PCR (qPCR) and citrate measurement, and dried samples were used for determining the biomass and P concentration in roots and shoots. P was analyzed using the molybdenum blue method (Murphy and Riley Citation1962) by a spectrophotometer (Multiskan GO, Thermo Fisher Scientific, Waltham, MA, USA) at 712 nm after digesting tissue samples with H2SO4–H2O2 mixture.
2.4. Quantitative reverse transcription PCR
The total RNA of the frozen root tissues was extracted with Plant Total RNA Extraction Mini Kit (Favorgen Biotech, Ping-Tung, Taiwan). The quality and quantity of extracted RNA were checked using a microphotometer (NanoDrop, Thermo Fisher Scientific). Five hundred nanograms of total RNA was used for cDNA synthesis with ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan), and synthesized cDNA solution was diluted 10 times with distilled water for the assay. qPCR was conducted on a LightCycler 96 real-time PCR system (Roche Diagnostics, Basel, Switzerland) with THUNDERBIRD SYBR qPCR Mix (TOYOBO). PCR was conducted for 45 cycles with denaturation at 95°C for 10 sec, annealing at 60°C for 10 s, and extension at 72°C for 15 s after an initial preincubation step at 90°C for 1 min. Melting curve analysis with a gradient from 65 to 97°C was conducted at the end of the reaction. A gene encoding 40S ribosomal protein S8 was used as a housekeeping gene. Primer pairs are summarized in Table S1.
2.5. Determination of citrate concentration in root
The citrate extraction from root tissue was determined using a method (Kihara et al. Citation2003) with a minor modification. Briefly, the 50 mg of frozen root tissue was homogenized in 0.4 mL of 0.6 M HClO4. After centrifuging for 5 min at 15,000 g and 4°C, 0.3 mL supernatant was neutralized by 20 µL of 5 M K2CO3. The solution was further centrifuged for 5 min at 15,000 g and 4°C, and the supernatant containing organic acids was stored at −20°C. The citrate concentration in extracts was determined using enzyme assay kits for citric acids (F-kit, Roche Diagnostics) using a spectrophotometer (Multiskan GO) at 340 nm. Then, this concentration was calculated following the manufacturer’s instructions (Wasaki et al. Citation2018).
2.6. Statistical analysis
Statistical comparisons were conducted using one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test (p < 0.05). Means are presented with the standard error.
3. Results
3.1. Induction of ethylene synthesis genes in CR development
The expression patterns of genes involved in ethylene synthesis and metabolism in the CRs of white lupin were investigated. Previously, Secco et al. (Citation2014) analyzed the transcriptome of CR at the different developmental stages in white lupin: the tip of CR (TCR), immature CR (ICR), mature CR (MCR), and senescent CR (SCR) (see Materials and Methods, and ), and their results propose that comprehensive transcriptional regulation is stimulated in CR and contributes to an adaptation to P deficiency in white lupin. In their paper, the authors focused on genes involved in P acquisition, metabolic regulation, and root development, including auxin-related genes; however, the involvement of ethylene-related genes in CR function and development were undescribed. Recently, the high-quality white lupin genome sequence and transcriptome information, including functional gene annotations, have been released (Hufnagel et al. Citation2020). Therefore, the RNA-Seq data published by Secco et al. (Citation2014) were reanalyzed with the up-to-date genome and transcriptome information to investigate the expression pattern of ethylene-related genes.
A total of 30,076 genes were identified in the CRs (TCR, ICR, and MCR). The analysis of differential gene expression enabled us to identify 5,740 and 541 DEGs (q < 0.05, |FC| ≥ 2) in comparison between TCR and MCR as well as between TCR and ICR, respectively (Fig. S1). Genes involved in P acquisition, such as phosphate transporters (LaPT1, LaPT2), an acid phosphatase (LaSAP2), an organic acid transporter (LaALMT2), and a citrate synthase (Lalb_Chr20g0108201: a gene with high homology to mitochondrial citrate synthase; Takita et al. Citation1999) were included in the up-regulated genes in MCR (Table S2). Additionally, it was confirmed that several auxin-related genes, such as 11 small auxin-up RNA (SAUR) family genes, 16 AUX-IAA family genes, and 16 ARF family genes exhibited higher expression in TCR than in MCR (Table S3).
The expression levels of three ACC oxidase genes and an ACC synthase gene, which are responsible for ethylene synthesis, were significantly higher in MCR than in TCR (Table S3), and these expressions were induced along with the maturation of CRs (Fig. S2). To validate the RNA-Seq data, the qPCR analyses of ACC oxidase1 (Lalb_Chr20g0119141), and ACC synthase1 (Lalb_Chr05g0224501) were conducted using five biological replicates. These expression patterns were consistent with the results of RNA-Seq analysis, which was up-regulated in the CRs maturation-dependent manner ().
3.2. Elongation of CR rootlet by an ethylene synthesis inhibitor
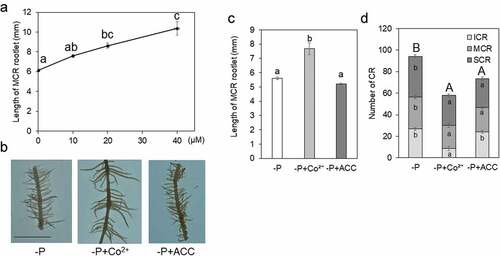
The role of ethylene in the CRs development and function was investigated using ethylene synthesis inhibitor Co2+ (Palit, Sharma, and Talukder Citation1994) and ethylene precursor ACC (Adams and Yang Citation1979). The application of Co2+ (CoCl2) dose-dependently promoted the elongation of CR rootlets (). The length of MCR rootlets under 30 µM Co2+ treatment was significantly longer than that under the control condition, while that under 0.1 µM ACC treatment was same with, or tended to be shorter than that under control (). The total number of CRs was reduced in Co2+ and ACC treatments, especially in the Co2+ treatment, compared with that in control (), and root biomass tended to be decreased by Co2+ and ACC treatments (), indicating that these treatments did not increase root growth. Those results suggest that ethylene inhibits the elongation of rootlets of CRs.
Table 1. Plant biomass and P concentration in shoot and root after Co2+ and 1-aminocyclopropane-1-carboxylic acid (ACC) treatments. Different letters indicate statistically significant differences (p < 0.05; Tukey-Kramer) among treatments (n = 5).
Figure 2. Cluster roots (CRs) influenced by reagent related to ethylene.
3.3. Effects of an ethylene synthesis inhibitor and an ethylene precursor on gene expression related to ethylene response and P acquisition, as well as citrate concentration in root
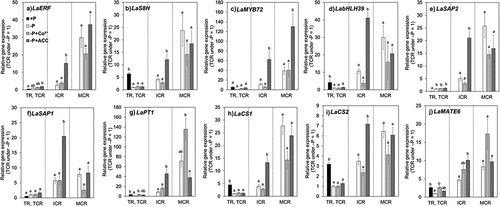
The expression of LaERF (Lalb_Chr03g0037181), LaS8H (Lalb_Chr01g0015881), LaMYB72 (Lalb_Chr20g0108581), and LabHLH39 (Lalb_Chr25g0286951), which are putative orthologs of AtERF1 (AT3G23240), AtS8H (AT3G12900), AtMYB72 (AT1G56160), and AtbHLH39 (AT3G56980) induced by ethylene, respectively (Cheng et al. Citation2013; Li and Lan Citation2017), were determined. The expression level of all four genes was significantly increased by ACC treatment in ICR (). Also, LaMYB72 expression was significantly up-regulated by ACC treatment in MCR (), although there were no significant differences in expression levels of LaERF, LaS8H, and LabHLH39. On the other hand, the expression of LaERF, LaS8H, and LabHLH39 tended to be down-regulated by Co2+ treatment in MCR (). And the expression of LaS8H, LaMYB72, and LabHLH39 tended to be down-regulated by Co2+ treatment in ICR (). These results suggest that ethylene accumulation in CR is induced by ACC treatment and suppressed by Co2+ treatment.
Figure 3. Relative gene expression for a)LaERF, b)LaS8H, c)LaMYB72, d)LabHLH39, e)LaSAP2, f)LaSAP1, g)LaPT1, h)LaCS1, i)LaCS2, and j)LaMATE6 in roots.
To investigate the effect of ethylene biosynthesis on the physiological function of CRs, the expression of genes related to P acquisition was determined in the CRs under Co2+ and ACC treatments. Eight genes for this investigation were selected: LaSAP1 (Lalb_Chr19g0140321) and LaSAP2 (Lalb_Chr20g0117931) encoding purple acid phosphatases; LaALMT1 (Lalb_Chr06g0168631) encoding a malate transporter and the homolog LaALMT2 (Lalb_Chr02g0149651); LaPT1 (Lalb_Chr10g0094631) and LaPT2 (Lalb_Chr04g0259111) encoding phosphate transporters; LaCS1 (Lalb_Chr18g0056171) and LaCS2 (Lalb_Chr20g0108201) encoding citrate synthases; LaMATE3 (Lalb_Chr25g0283091) and LaMATE6 (Lalb_Chr02g0156391) encoding putative citrate transporters. All the tested genes were up-regulated in MCR (, S3, and S4).
LaSAP2 is a primary secretory acid phosphatase of white lupin induced by P deficiency, especially in CRs (Wasaki et al. Citation2000, Citation2003). The expression level under the P-deficient condition was increased during CR maturation and reached to the highest level in MCR (), supporting a previous finding that the activity of secretory acid phosphatase is the highest in MCR among the different CR developmental stages (Wasaki et al. Citation2003, Citation2018). LaSAP2 expression level in MCR tended to be lower under Co2+ treatment than under the untreated control (). Alternatively, LaSAP2 expression level was significantly increased by ACC treatment in ICR, and maintained in MCR. These results suggest relationship between the accumulation of ethylene and the maturation-dependent LaSAP2 expression. LaSAP1, the acid phosphatase localized at the cell membrane (Wasaki et al. Citation1999), also showed increased expression by ACC treatment in ICR, and tended to be down-regulated by Co2+ treatment in MCR ().
LaPT1 is one of the putative phosphate transporter genes induced in response to P deficiency and up-regulated during CR development (Le Thanh et al. Citation2021). The expression of LaPT1 was up-regulated by ACC treatment compared with that in the untreated control in ICR (), suggesting that the expression level of LaPT1 was influenced by ethylene accumulation as is in the case of acid phosphatase genes. But LaPT1 expression level was also increased by Co2+ treatment in MCR compared with that in the untreated control (), suggesting that the ethylene accumulation at a high level rather suppresses P absorption by LaPT1 in CR.
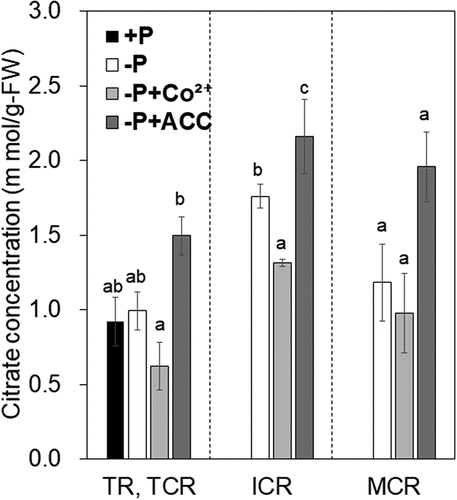
Generally, plants synthesize and secrete citrate from roots to mobilize P in low P soils (Kihara et al. Citation2003), and CR of lupin has the high ability to secrete citrate (Tiziani et al. Citation2021). LaCS1 and LaCS2, the citrate synthase homolog genes, were up-regulated during the maturation of CR, according to RNA-Seq transcriptomic analysis (Fig. S3). The expression of LaCS1 and LaCS2 was significantly induced by ACC treatment in ICR, and the induction was maintained in MCR. In contrast, those expressions tended to be down-regulated by Co2+ treatment compared with that in the untreated control (). Accordingly, citrate concentration in CR tended to be increased under ACC treatment and reduced under Co2+ treatment, respectively (). These results support that the synthesized ethylene induces citrate synthesis in CR.
Figure 4. Effects of Co2+ and 1-aminocyclopropane-1-carboxylic acid (ACC) on citrate concentration in roots.
The genome of white lupin harbors eight genes of the multidrug efflux transporter family with Citrate Exuding Motif (Yang et al. Citation2011). LaMATE6 (Zhou et al. Citation2021) exhibited the induction during CR development and the largest log2|FC| in comparison between TCR and MCR among the paralogous genes following the transcriptomic data (Table S2), suggesting that LaMATE6 influences citrate secretion in CR. qPCR confirmed the induction of LaMATE6 during the maturation of CR (). LaMATE6 expression was significantly increased by ACC treatment in ICR compared with that in the untreated control (), suggesting that ethylene induces LaMATE6 expression and expression of genes induced in the CR described above. However, LaMATE6 expression was also up-regulated in Co2+-treated MCR (). This suggests that citrate efflux is further promoted in the presence of Co2+, though ethylene biosynthesis is inhibited.
4. Discussion
4.1. Ethylene arrests rootlet elongation
DEGs up-regulated in MCR contained some P-starvation-responsive genes, while DEGs up-regulated in TCR contained some auxin-related genes (Tables S2 and S3), in accordance with previous analyses (Secco et al. Citation2014; Wang et al. Citation2014). Some genes involved in ethylene synthesis were up-regulated in MCR according to transcriptomic analysis and quantitative gene expression ( and S2), which suggests that ethylene affected CR morphology. Indeed, the application of an inhibitor for ethylene synthesis increased the length of rootlets in MCR (). Alternatively, the length of rootlets in MCR under ethylene precursor application tended to be shorter than that of the control, although it was not significant ().
In agreement with this study, some previous studies have reported that exogenous ACC inhibited the elongation of lateral roots in Arabidopsis and rice (Ivanchenko, Muday, and Dubrovsky Citation2008; Malheiros et al. Citation2019; Negi, Ivanchenko, and Muday Citation2008; Strader, Chen, and Bartel Citation2010). Malheiros et al. (Citation2019) showed that the elongation of primary root in rice was promoted in the presence of an ethylene synthesis inhibitor aminoethoxyvinylglycine (AVG). At the initial phase of lateral root formation in Arabidopsis, Lewis et al. (Citation2011) reported that ACC application enhanced the expression of auxin efflux transporter genes, such as PIN3 and PIN7, in an ETR1 and EIN2 (ethylene signaling)-dependent manner. The enhancement of these auxin effluxes contributed to preventing localized auxin accumulation to form lateral roots, while AVG application had opposite effects on roots (Lewis et al. Citation2011). Therefore, ethylene signaling would connect with auxin transport to suppress rootlet elongation in CR, although further experiments are required.
In terms of the number of CRs (), decreasing them implies that internal ethylene concentration is important for initiating CR development. The ability to form new CRs is limited in the absence of ethylene. Wang et al. (Citation2015) reported that CR was hardly detected by 10 µM CoCl2 application to roots before forming CRs in white lupin. While, decreased number of CRs under ACC treatment could be caused by senescence and inhibiting the growth of the whole root by ACC application. Fukaki and Tasaka (Citation2009) summarized that ethylene plays an essential role in regulating the initiation and elongation of lateral roots in Arabidopsis. We suggest the involvement of ethylene to emerge rootlets in the initial stage and to arrest rootlet elongation in the mature stage. The characteristics of CRs could be specified as (1) multiple crowding rootlets and (2) short rootlets, we hint on (2) that ethylene makes rootlets short in CRs.
4.2. Ethylene promotes P acquisition under P-deficient conditions
It is suggested that ethylene synthesis in CR is involved in the transcriptional regulation of genes important for CR function, such as genes encoding acid phosphatases, phosphate transporters, citrate synthases, and a putative citrate transporter. Ramaiah, Jain, and Raghothama (Citation2014) reported that overexpression of Arabidopsis thaliana ethylene response factor070 (AtERF070) changed the expression of a subset of P starvation-induced genes containing miRNA399b and miRNA399f in root. miRNA399s induced by P deficiency regulate multiple-gene expressions related to P absorption or P translocation from root to shoot through post-transcriptional repression of phosphate2 (PHO2) (Liu et al. Citation2014). Additionally, ethylene synthesis is improved in different nutrient-deficient conditions, which induces the expression of nutrient-absorbing transporters (Chapin and Jones Citation2009; Jiang et al. Citation2020; Jung, Shin, and Schachtman Citation2009; Li et al. Citation2011; Lucena et al. Citation2006). This is a P starvation-response independent of the maturation- or senescence-inducing effect, which may affect enhancing P absorption during the maturation of CR.
The sensitivity to ethylene depends on the genes. The expression of AtNRT1.1 encoding a nitrate transporter was increased by ACC application to Arabidopsis roots under N-deficient conditions, while that of AtNRT2.1 was reduced (Tian, Sun, and Zhang Citation2009). Furthermore, AVG application reduced and increased the expression of AtNRT1.1 and AtNRT1.2 in roots under N-sufficient condition, respectively, which was opposite to the response to ACC application (Tian, Sun, and Zhang Citation2009). This result may be because of the different ethylene sensitivity between these two genes, suggesting that ethylene can act as a promoter or a repressor in nutrient transporters, depending on the concentration. In this study, ethylene synthesis in MCR would be responsible for suppressing the expression of specific genes, such as LaPT1 and LaMATE6. Additionally, the other genes (LaALMT1, LaALMT2, LaPT2, and LaMATE3) were grossly inhibited by Co2+ and ACC application (Fig. S4) due to excessive ethylene sensitivity, although it may be involved in transcriptional regulation.
Another possibility is that the moderately long-term (9 days) treatment may have resulted in changes in expression levels during rootlets elongation. For example, secretory acid phosphatase activities were enhanced at meristematic region of rootlet corresponding to that of lateral roots (Wasaki et al. Citation2008), and LASAP2 occupies a large proportion of acid phosphatase acting in the rhizosphere of white lupin (Wasaki et al. Citation2008), so that localization of LASAP2 would be root tips of rootlets. Longer rootlets caused by Co2+ treatment may reduce the relative mRNA accumulation of LaSAP2 per CRs, even though it was accumulated in same level on tips of each rootlet. Short rootlets would contribute to mRNA enrichment per CR, though it depends on the localization. Further elucidation of the localization sites of other proteins will provide further insight into expression variation in this study. Primary metabolism under P-deficient conditions in plants is regulated to adapt to low P stress. For example, white lupin placed in the P-deficient condition promotes the synthesis and accumulation of citrate for P acquisition (Kihara et al. Citation2003). In this study, the expression of genes encoding citrate synthase exhibited a similar trend to citrate concentration in root (), which suggests that ethylene synthesis enhances citrate synthesis. Alternatively, in terms of the citrate transport, the expression of LaMATE6 in Co2+-treated MCR tended to be opposite to that of LaCS1 and LaCS2 (), suggesting that the internal ethylene concentration for induction of LaMATE6 transcription is lower than that of LaCS1 and LaCS2. Neumann et al. (Citation1999) reported that citrate secretion, which is most active in the mature stage of CR, was markedly inhibited during aging. The expression of LaMATE6 is transiently induced in the pre-mature stage in CR and subsequently repressed by ethylene synthesis in MCR, implying that modulating ethylene sensitivity of LaMATE6 still improves citrate secretion in MCR.
The reduction of citrate concentration in MCR could be caused by citrate secretion. The fact that citrate concentration in ACC-treated MCR tended to be higher than that in the untreated control may be due to the suppression of secretion by sensing as described by Neumann et al. (Citation1999).
Although transcript levels of LaCS1 and LaCS2 in the TR under P-sufficient conditions were higher than that of untreated TCR (, the citrate concentration was not increased (). Under P-sufficient conditions, the activities of not only citrate synthase but also NADP-isocitrate dehydrogenase are higher than those under P-deficient conditions (Kihara et al. Citation2003), which contributes to inhibiting citrate accumulation in roots. In this study, the effect of ethylene on citrate accumulation was recognized on the basis of the transcript level of citrate synthase. However, various other factors induced under P deficiency have been reported to be involved in citrate accumulation (Johnson, Allan, and Vance Citation1994; Kihara et al. Citation2003). Investigation of the expression or activities of these factors involved in citrate accumulation would elucidate an accurate mechanism: how ethylene accumulates citrate in CR.
In conclusion, the strengths of ethylene signaling in roots were changed by application of an ethylene synthesis inhibitor (Co2+) and an ethylene precursor (1-aminocyclopropane-1-carboxylic acid; ACC) to root directly. We conclude that there are two kinds of roles of ethylene in CR. One is the suppression of rootlets elongation to form short rootlets. Ethylene signaling may connect with auxin transport to suppress rootlet elongation in CR, although further experiments are required. The other is the induction of genes for P acquisition, such as LaSAP1 and LaSAP2 encoding purple acid phosphatases; LaPT1 encoding a phosphate transporter; LaCS1 and LaCS2 encoding citrate synthases; and LaMATE6 encoding a putative citrate transporter. The citrate concentration in root was similar to the expression pattern of citrate synthases, suggesting that ethylene works as a possible regulator for phosphorus acquisition in CR of white lupin. In addition, short rootlets would contribute to mRNA enrichment per CR, though it depends on the localization ().
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abel, S., C. A. Ticconi, and C. A. Delatorre. 2002. “Phosphate Sensing in Higher Plants.” Physiologia Plantarum 115 (1): 1–8. doi:10.1034/j.1399-3054.2002.1150101.x.
- Adams, D., and S. F. Yang. 1979. “Ethylene Biosynthesis-Identification of 1-Aminocyclopropane-1-Carboxylic Acid as an Intermediate in the Conversion of Methionine to Ethylene.” Proceedings of the National Academy of Sciences of the USA 76 (1): 170–174. doi:10.1073/pnas.76.1.170.
- Bichara, S., P. Mazzafera, and S. A. L. de Andrade. 2021. “Root Morphological Changes in Response to Low Phosphorus Concentration in Eucalypt Species.” Trees-Structure and Function 35 (6): 1933–1943. doi:10.1007/s00468-021-02161-4.
- Chapin, L. J., and M. L. Jones. 2009. “Ethylene Regulates Phosphorus Remobilization and Expression of a Phosphate Transporter (Phpt1) during Petunia Corolla Senescence.” Journal of Experimental Botany 60 (7): 2179–2190. doi:10.1093/jxb/erp092.
- Cheng, M. C., P. M. Liao, W. W. Kuo, and T. P. Lin. 2013. “The Arabidopsis Ethylene Response Factor1 Regulates Abiotic Stress-Responsive Gene Expression by Binding to Different Cis-Acting Elements in Response to Different Stress Signals.” Plant Physiology 162 (3): 1566–1582. doi:10.1104/pp.113.221911.
- Cordell, D., J. O. Drangert, and S. White. 2008. “The Story of Phosphorus: Global Food Security and Food for Thought.” Global Environmental Change-Human and Policy Dimensions 19 (2): 292–305. doi:10.1016/j.gloenvcha.2008.10.009.
- Du, Y. J., and B. Scheres. 2018. “Lateral Root Formation and the Multiple Roles of Auxin.” Journal of Experimental Botany 69 (2): 155–167. doi:10.1093/jxb/erx223.
- Fukaki, H., and M. Tasaka. 2009. “Hormone Interactions During Lateral Root Formation.” Plant Molecular Biology 69 (4): 437–449. doi:10.1007/s11103-008-9417-2.
- Gallardo, C., B. Hufnagel, C. Casset, C. Alcon, F. Garcia, F. Divol, L. Marques, P. Doumas, and B. Peret. 2019. “Anatomical and Hormonal Description of Rootlet Primordium Development along White Lupin Cluster Root.” Physiologia Plantarum 165 (1): 4–16. doi:10.1111/ppl.12714.
- Gilbert, G. A., J. D. Knight, C. P. Vance, and D. L. Allan. 2000. “Proteoid Root Development of Phosphorus Deficient Lupin Is Mimicked by Auxin and Phosphonate.” Annals of Botany 85 (6): 921–928. doi:10.1006/anbo.2000.1133.
- Hinsinger, P. 2001. “Bioavailability of Soil Inorganic P in the Rhizosphere as Affected by Root-Induced Chemical Changes: A Review.” Plant and Soil 237 (2): 173–195. doi:10.1023/A:1013351617532.
- Hufnagel, B., A. Marques, A. Soriano, L. Marques, F. Divol, P. Doumas, E. Sallet, D. Mancinotti, S. Carrere, and W. Marande. 2020. “High-Quality Genome Sequence of White Lupin Provides Insight into Soil Exploration and Seed Quality.” Nature Communications 11 (1): 492. doi:10.1038/s41467-019-14197-9.
- Ivanchenko, M. G., G. K. Muday, and J. G. Dubrovsky. 2008. “Ethylene-Auxin Interactions Regulate Lateral Root Initiation and Emergence in Arabidopsis Thaliana.” Plant Journal 55 (2): 335–347. doi:10.1111/j.1365-313X.2008.03528.x.
- Jiang, L., J. Yang, C. X. Liu, Z. P. Chen, Z. C. Yao, and S. Q. Cao. 2020. “Overexpression of Ethylene Response Factor ERF96 Gene Enhances Selenium Tolerance in Arabidopsis.” Plant Physiology and Biochemistry 149: 294–300. doi:10.1016/j.plaphy.2020.02.024.
- Johnson, J. F., D. L. Allan, and C. P. Vance. 1994. “Phosphorus Stress-Induced Proteoid Roots Show Altered Metabolism in Lupinus Albus.” Plant Physiology 104 (2): 657–665. doi:10.1104/pp.104.2.657.
- Jung, J. Y., R. Shin, and D. P. Schachtman. 2009. “Ethylene Mediates Response and Tolerance to Potassium Deprivation in Arabidopsis.” The Plant Cell 21 (2): 607–621. doi:10.1105/tpc.108.063099.
- Kihara, T., T. Wada, Y. Suzuki, T. Hara, and K. Koyama. 2003. “Alteration of Citrate Metabolism in Cluster Roots of White Lupin.” Plant & Cell Physiology 44 (9): 901–908. doi:10.1093/pcp/pcg115.
- Lambers, H., E. Martinoia, and M. Renton. 2015. “Plant Adaptations to Severely Phosphorus-Impoverished Soils.” Current Opinion in Plant Biology 25: 23–31. doi:10.1016/j.pbi.2015.04.002.
- Lambers, H., M. W. Shane, M. D. Cramer, S. J. Pearse, and E. J. Veneklaas. 2006. “Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits.” Annals of Botany 98 (4): 693–713. doi:10.1093/aob/mcl114.
- Lau, O. L., and S. F. Yang. 1976. “Inhibition of Ethylene Production by Cobaltous Ion.” Plant Physiology 58 (1): 114–117. doi:10.1104/pp.58.1.114.
- Le Thanh, T., B. Hufnagel, A. Soriano, F. Divol, L. Brottier, C. Casset, B. Peret, P. Doumas, and L. Marques. 2021. “Dynamic Development of White Lupin Rootlets along a Cluster Root.” Frontiers in Plant Science 12: 738172. doi:10.3389/fpls.2021.738172.
- Lei, M. G., C. M. Zhu, Y. D. Liu, A. S. Karthikeyan, R. A. Bressan, K. G. Raghothama, and D. Liu. 2011. “Ethylene Signalling Is Involved in Regulation of Phosphate Starvation-Induced Gene Expression and Production of Acid Phosphatases and Anthocyanin in Arabidopsis.” New Phytologist 189 (4): 1084–1095. doi:10.1111/j.1469-8137.2010.03555.x.
- Lewis, D. R., S. Negi, P. Sukumar, and G. K. Muday. 2011. “Ethylene Inhibits Lateral Root Development, Increases IAA Transport and Expression of PIN3 and PIN7 Auxin Efflux Carriers.” Development 138 (16): 3485–3495. doi:10.1242/dev.065102.
- Li, Y. S., Y. Gao, Q. Y. Tian, F. L. Shi, L. H. Li, and W. H. Zhang. 2011. “Stimulation of Root Acid Phosphatase by Phosphorus Deficiency Is Regulated by Ethylene in Medicago Falcata.” Environmental and Experimental Botany 71 (1): 114–120. doi:10.1016/j.envexpbot.2010.11.007.
- Li, W. F., and P. Lan. 2017. “The Understanding of the Plant Iron Responses in Strategy I Plants and the Role of Ethylene in This Process by Omic Approaches.” Frontiers in Plant Science 8: 40. doi:10.3389/fpls.2017.00040.
- Liu, T. Y., W. Y. Lin, T. K. Huang, and T. J. Chiou. 2014. “MicroRNA-Mediated Surveillance of Phosphate Transporters on the Move.” Trends in Plant Science 19 (10): 647–655. doi:10.1016/j.tplants.2014.06.004.
- Liu, Y., Y. R. Xie, H. Wang, X. J. Ma, W. J. Yao, and H. Y. Wang. 2017. “Light and Ethylene Coordinately Regulate the Phosphate Starvation Response through Transcriptional Regulation of Phosphate Starvation Response1.” The Plant Cell 29 (9): 2269–2284. doi:10.1105/tpc.17.00268.
- Locke, J. M., J. H. Bryce, and P. C. Morris. 2000. “Contrasting Effects of Ethylene Perception and Biosynthesis Inhibitors on Germination and Seedling Growth of Barley (Hordeum Vulgare L.).” Journal of Experimental Botany 51 (352): 1843–1849. doi:10.1093/jexbot/51.352.1843.
- Lucena, C., B. M. Waters, F. J. Romera, M. J. Garcia, M. Morales, E. Alcantara, and R. Perez-Vicente. 2006. “Ethylene Could Influence Ferric Reductase, Iron Transporter, and H+-ATPase Gene Expression by Affecting FER (Or FER-like) Gene Activity.” Journal of Experimental Botany 57 (15): 4145–4154. doi:10.1093/jxb/erl189.
- Malheiros, R. S. P., L. C. Costa, R. T. Avila, T. M. Pimenta, L. S. Teixeira, F. A. L. Brito, A. Zsogon, W. L. Araujo, and D. M. Ribeiro. 2019. “Selenium Downregulates Auxin and Ethylene Biosynthesis in Rice Seedlings to Modify Primary Metabolism and Root Architecture.” Planta 250 (1): 333–345. doi:10.1007/s00425-019-03175-6.
- Murphy, J., and J. P. Riley. 1962. “A Modified Single Solution Method for the Determination of Phosphate in Natural Waters.” Analytic Chemical Acta 27: 31–36. doi:10.1016/S0003-2670(00)88444-5.
- Negi, S., M. G. Ivanchenko, and G. K. Muday. 2008. “Ethylene Regulates Lateral Root Formation and Auxin Transport in Arabidopsis Thaliana.” The Plant Journal 55 (2): 175–187. doi:10.1111/j.1365-313X.2008.03495.x.
- Neumann, G., and E. Martinoia. 2002. “Cluster Roots – An Underground Adaptation for Survival in Extreme Environments.” Trends in Plant Science 7 (4): 162–167. doi:10.1016/S1360-1385(02)02241-0.
- Neumann, G., A. Massonneau, E. Martinoia, and V. Romheld. 1999. “Physiological Adaptations to Phosphorus Deficiency during Proteoid Root Development in White Lupin.” Planta 208 (3): 373–382. doi:10.1007/s004250050572.
- Nishida, S., Y. Kakei, Y. Shimada, and T. Fujiwara. 2017. “Genome-Wide Analysis of Specific Alterations in Transcript Structure and Accumulation Caused by Nutrient Deficiencies in Arabidopsis Thaliana.” The Plant Journal 91 (4): 741–753. doi:10.1111/tpj.13606.
- Palit, S., A. Sharma, and G. Talukder. 1994. “Effects of Cobalt on Plants.” Botanical Review 60 (2): 149–181. doi:10.1007/BF02856575.
- Peret, B., M. Clement, L. Nussaume, and T. Desnos. 2011. “Root Developmental Adaptation to Phosphate Starvation: Better Safe than Sorry.” Trends in Plant Science 16 (8): 442–450. doi:10.1016/j.tplants.2011.05.006.
- Ramaiah, M., A. Jain, and K. G. Raghothama. 2014. “Ethylene Response Factor070 Regulates Root Development and Phosphate Starvation-Mediated Responses.” Plant Physiology 164 (3): 1484–1498. doi:10.1104/pp.113.231183.
- Schachtman, D. P., R. J. Reid, and S. M. Ayling. 1998. “Phosphorus Uptake by Plants: From Soil to Cell.” Plant Physiology 116 (2): 447–453. doi:10.1104/pp.116.2.447.
- Secco, D., H. X. Shou, J. Whelan, and O. Berkowitz. 2014. “RNA-Seq Analysis Identifies an Intricate Regulatory Network Controlling Cluster Root Development in White Lupin.” BMC Genomics 15: 230. doi:10.1186/1471-2164-15-230.
- Shane, M. W., M. D. Cramer, S. Funayama-Noguchi, G. R. Cawthray, A. H. Millar, D. A. Day, and H. Lambers. 2004. “Development Physiology of Cluster-Root Carboxylate Synthesis and Exudation in Harsh Hakea. Expression of Phosphoenolpyruvate Carboxylase and the Alternative Oxidase.” Plant Physiology 135 (1): 549–560. doi:10.1104/pp.103.035659.
- Skene, K. R. 2000. “Pattern Formation in Cluster Roots: Some Developmental and Evolutionary Considerations.” Annals of Botany 85 (6): 901–908. doi:10.1006/anbo.2000.1140.
- Song, L., and D. Liu. 2015. “Ethylene and Plant Responses to Phosphate Deficiency.” Frontiers in Plant Science 6: 796. doi:10.3389/fpls.2015.00796.
- Song, L., H. P. Yu, J. S. Dong, X. M. Che, Y. L. Jiao, and D. Liu. 2016. “The Molecular Mechanism of Ethylene-Mediated Root Hair Development Induced by Phosphate Starvation.” PLoS Genetics 12 (7): e1006194. doi:10.1371/journal.pgen.1006194.
- Strader, L. C., G. L. Chen, and B. Bartel. 2010. “Ethylene Directs Auxin to Control Root Cell Expansion.” The Plant Journal 64 (5): 874–884. doi:10.1111/j.1365-313X.2010.04373.x.
- Takita, E., H. Koyama, Y. Shirano, D. Shibata, and T. Hara. 1999. “Structure and Expression of the Mitochondrial Citrate Synthase Gene in Carrot Cells Utilizing Al-Phosphate.” Soil Science and Plant Nutrition 45 (1): 197–205. doi:10.1080/00380768.1999.10409335.
- Tang, H. L., X. Q. Li, C. Zu, F. S. Zhang, and J. B. Shen. 2013. “Spatial Distribution and Expression of Intracellular and Extracellular Acid Phosphatase of Cluster Roots at Different Developmental Stages in White Lupin.” Journal of Plant Physiology 170 (14): 1243–1250. doi:10.1016/j.jplph.2013.04.015.
- Tian, Q. Y., P. Sun, and W. H. Zhang. 2009. “Ethylene Is Involved in Nitrate-Dependent Root Growth and Branching in Arabidopsis Thaliana.” New Phytologist 184 (4): 918–931. doi:10.1111/j.1469-8137.2009.03004.x.
- Tiziani, R., M. Puschenreiter, E. Smolders, T. Mimmo, J. C. Herrera, S. Cesco, and J. Santner. 2021. “Millimetre-Resolution Mapping of Citrate Exuded from Soil-grown Roots Using a Novel, Low-Invasive Sampling Technique.” Journal of Experimental Botany 72 (10): 3513–3525. doi:10.1093/jxb/erab123.
- Vance, P. C., C. Uhde-Stone, and D. L. Allan. 2003. “Phosphorus Acquisition and Use: Critical Adaptations by Plants for Securing a Nonrenewable Resource.” New Phytologist 157 (3): 423–447. doi:10.1046/j.1469-8137.2003.00695.x.
- Wang, Z. R., A. B. M. M. Rahman, G. Y. Wang, U. Ludewig, J. B. Shen, and G. Neumann. 2015. “Hormonal Interactions during Cluster-Root Development in Phosphate-Deficient White Lupin (Lupinus Albus L.).” Journal of Plant Physiology 117: 74–82. doi:10.1016/j.jplph.2014.10.022.
- Wang, Z. R., D. Straub, H. Y. Yang, A. Kania, J. B. Shen, U. Ludewig, and G. Neumann. 2014. “The Regulatory Network of Cluster-Root Function and Development in Phosphate-Deficient White Lupin (Lupinus Albus) Identified by Transcriptome Sequencing.” Physiologia Plantarum 151 (3): 323–338. doi:10.1111/ppl.12187.
- Wasaki, J., S. Dojima, H. Maruyama, S. Haase, M. Osaki, and E. Kndeler. 2008. “Localization of Acid Phosphatase Activities in the Roots of White Lupin Plants Grown under Phosphorus-Deficient Conditions.” Soil Science and Plant Nutrition 54 (1): 95–102. doi:10.1111/j.1747-0765.2007.00207.x.
- Wasaki, J., M. Omura, M. Ando, H. Dateki, T. Shinan, M. Osaki, H. Ito, H. Matsui, and T. Tadano. 2000. “Molecular Cloning and Root Specific Expression of Secretory Acid Phosphatase from Phosphate Deficient Lupin (Lupinus Albus L.).” Soil Science and Plant Nutrition 46 (2): 427–437. doi:10.1080/00380768.2000.10408796.
- Wasaki, J., M. Omura, M. Osaki, H. Ito, H. Matsui, T. Shinano, and T. Tadano. 1999. “Structure of a cDNA for an Acid Phosphatase from Phosphate-Deficient Lupin (Lupinus Albus L.) Roots.” Soil Science and Plant Nutrition 45 (2): 439–449. doi:10.1080/00380768.1999.10409358.
- Wasaki, J., J. Sakaguchi, T. Yamamura, S. Ito, T. Shinano, M. Osaki, and E. Kandeler. 2018. “P and N Deficiency Change the Relative Abundance and Function of Rhizosphere Microorganisms during Cluster Root Development of White Lupin (Lupinus Albus L.).” Soil Science and Plant Nutrition 64 (6): 686–696. doi:10.1080/00380768.2018.1536847.
- Wasaki, J., T. Yamamura, T. Shinano, and M. Osaki. 2003. “Secreted Acid Phosphatase Is Expressed in Cluster Roots of Lupin in Response to Phosphorus Deficiency.” Plant and Soil 248 (1–2): 129–136. doi:10.1023/A:1022332320384.
- Watt, M., and J. R. Evans. 2003. “Phosphorus Acquisition from Soil by White Lupin (Lupinus Albus L.) And Soybean (Glycine Max L.), Species with Contrasting Root Development.” Plant and Soil 248 (1–2): 271–283. doi:10.1023/A:1022332700686.
- Yang, X. Y., J. L. Yang, Y. Zhou, M. A. Pineros, L. V. Kochian, G. X. Li, and S. J. Zheng. 2011. “A de Novo Synthesis Citrate Transporter, Vigna Umbellata Multidrug and Toxic Compound Extrusion, Implicates in Al-Activated Citrate Efflux in Rice Bean (Vigna Umbellata) Root Apex.” Plant, Cell & Environment 34 (12): 2138–2148. doi:10.1111/j.1365-3040.2011.02410.x.
- Zhou, Y. P., P. Olt, B. Neuhauser, N. Moradtalab, W. Bautista, C. Uhde-Stone, G. Neumann, and U. Ludewig. 2021. “Loss of LaMATE Impairs Isoflavonoid Release from Cluster Roots of Phosphorus-Deficient White Lupin.” Physiologia Plantarum 173 (3): 1207–1220. doi:10.1111/ppl.13515.