ABSTRACT
One of the most entrenched binary oppositions in archaeology and anthropology has been the agriculturalist vs hunter-gatherer-fisher dichotomy fuelling a debate that this paper tackles from the bottom-up by seeking to reconstruct full past diets. The Japanese prehistoric Jōmon cultures survived without fully-developed agriculture for more than 10,000 years. Here we compile a comprehensive, holistic database of archaeobotanical and archaeozoological records from the two ends of the archipelago, the northernmost prefecture of Hokkaido and the southernmost island-chain of Ryukyu. The results suggest Jōmon diets varied far more geographically than they did over time, and likely cultivated taxa were important in both regions. This provides the basis for examining how fisher-hunter-gatherer diets can fulfil nutritional requirements from varied environments and were resilient in the face of environmental change.
1. Introduction
One of the most deeply entrenched, enduring, and binary oppositions in archaeology has been the agriculturalist vs hunter-gatherer-fisher dichotomy (Cummings Citation2014). Whilst justifiable in some regions, it has been an underlying tension in the debates of archaeological scientists and anthropologists. The echoes of Childe’s Neolithic revolution and its associations with sedentism, hierarchy, and pottery have been hard to shed. In this context, the Jomon cultures of the Japanese archipelago epitomize the debate being variously regarded as ‘hunter-gatherers-fishers (hgfs)’, ‘affluent hgfs’, ‘complex hgfs’, and ‘hunter-cultivators’ (Habu Citation2004; Obata, Miyaura, and Nakano; Pearson Citation2006). Unfortunately, the debate has been polarised and requires a bottom-up approach, which is the basis of the project outlined here. The large archive of palaeoeconomic data from development-driven archaeological excavations provides a comprehensive picture of the regional and temporal variations in the diet of Jomon societies, which can be interpreted free from preconceptions concerning the relationships between subsistence and other aspects of material culture. Rather than using unavoidably vague and loaded descriptors of societies’ subsistence base, analyses should be founded on as comprehensive as possible dietary reconstructions allowing robust comparisons with cultural and skeletal data on health and well-being.
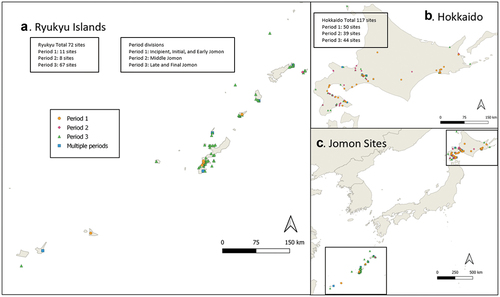
Jōmon cultures lasted for over 10,000 years (c. 16,000–2,800/2,300 cal. BP) and adapted to environmental change throughout a wide range of climatic zones but without fully-developed agriculture (Kitagawa et al. Citation2018; Kusaka, Yamada, and Yoneda Citation2018). Although outside contacts were minimal for most of this period, after c. 3,500 BP both archaeological and linguistic data suggest some contacts prior to the arrival of fully-developed agriculture c. 2,900 BP (Hudson et al. Citation2021). The study of this cultural adaptability can shed light on major archaeological issues such as both the geographical and temporal variability of resources and impacts on foodways and social systems (Robson et al. Citation2020). The distribution of the Jomon over four biomes; Subarctic, Cold temperate, Warm temperate and Subtropical (Shimizu Citation2014) provides the opportunity to evaluate how predominantly non-agricultural cultures adapted to environmental change in different geographical contexts and how this was reflected in nutritional variation over time and space. To do this, we have compiled a comprehensive database of archaeobotanical and archaeozoological remains from Jōmon sites from the two most biogeographically different areas in the archipelago, the Ryukyu Islands (south) and Hokkaido Island (north) (; Tables S1 and S2).
Figure 1. The distribution of Jōmon sites included in the database. (a) Jōmon sites in Ryukyu. (b) Jōmon sites in Hokkaido. (c) All Jōmon sites included in the database reported here. The three most southerly sites in the Ryukyu Islands are from the Sakashima Islands. Although not formerly Jōmon, they provide comparable pre-agricultural Neolithic sites.
2. Jōmon culture, environments, and diet
The Jōmon period is divided into six phases (Table S3) based on pottery typology (Habu Citation2004). There is a general trend of increasing population from the Incipient to Middle Jōmon, followed by a decline (Crema et al. Citation2016; Koyama and Sugito Citation1984). However, there were geographical differences, and the decreased population in the late-Middle Jōmon, attributed to over-specialization of subsistence (Habu Citation2015; Hudson et al. Citation2021), may be a geographically restricted phenomenon (Crema et al. Citation2016). Jōmon populations were sustained through a diverse diet with hunting, gathering, and fishing, with some plant management (Crawford and Takamiya Citation2008; Nakayama Citation2015; Obata, Sasaki, and Senba). However, since both terrestrial and marine resources varied geographically, it follows that subsistence strategies were locally adapted (Takahashi, Toizumi, and Kojo). Jōmon environments changed at the end of the Pleistocene and during the Holocene (Morley, Heusser, and Sarro Citation1986). Rapid warming at the Pleistocene-Holocene boundary was accompanied by rising relative sea levels (RSL) and increased biotic productivity (Aikens and Akazawa Citation1996). The postglacial Jōmon transgression had several oscillations with a major relative sea-level fall around 11,000–10,000 BP and maximum transgression around 7,000 BP (Umitsu Citation1991). RSL is important because it changes significantly the land-area available for settlement, and it affects local marine resources (Abe et al. Citation2016). Currents around Japan have influenced local climate, coastal ecosystems and resource availability (Morioka, Varlamov, and Miyazawa Citation2019) (). Japan was a forested environment during the Jomon period as it remains today (67%) (MAFF (Ministry of Agriculture, Forestry and Fisheries)) with forest zones reflecting temperature and precipitation (Gotanda et al. Citation2002).
Figure 2. Ocean currents, biogeographical boundaries, and current and past vegetations of Japan adapted from Takahara et al. (Citation2000) and Gotanda et al. (Citation2002). (a) Location map of Japan. (b) Current vegetation map of Japan. (c) Vegetation around 2,500 BP. (d) Vegetation around 6,000 BP. (e) Vegetation around 18,000 BP.
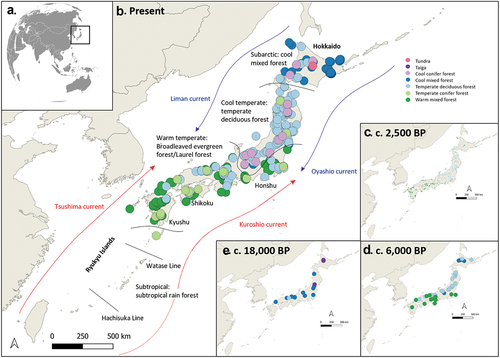
The Biome gradient (; Table S3), which varied S-N from cool mixed (Boreal) forest to taiga in the Incipient Jōmon, through warm mixed forest to cool mixed forest (Boreal) in the middle Jōmon, varies from subtropical rain forest to cool mixed forest (Boreal) today (Ooi Citation2016).
Due to both warm and cold currents surrounding the archipelago, a high diversity of marine taxa were, and remain, present. Along the coast, fish and shellfish are abundant (3,700 and 9,000 species, respectively), and they have been historically a major protein source (Fujikura et al. Citation2010; JBOSC (Japan Biodiversity Outlook Science Committee, The Ministry of the Environment) Citation2010). Many of the species present have been found on Jōmon sites (Table S4 and S5). Ryukyu has around 5,200 species of shellfish (60% of shellfish in Japan) (Kubo Citation2017) whereas the eastern side of Hokkaido has c. 300 species of intertidal shellfish (Yamazaki). Marine mammals are common with species including northern sea lions (Eumetopias jubatus) and northern fur seals (Callorhinus ursinus) in Hokkaido and dugongs (Dugong dugon) in Ryukyu (GBIF.org Citation2021) (Table S6). There have been significant changes during the Holocene (Yamano, Sugihara, and Nomura Citation2011). Subtropical species of fish such as catfish (Plicofollis nella) and croakers (Nibea albiflora) excavated from sites occupied during the Jōmon transgression in western Japan are not present there today (Ohe, Tajima, and Shinkai Citation2016). Shellfish during the transgression were also different from those today in some areas (Matsushima). The distribution of the mammalian, reptilian, and amphibian species also reflects the biogeographic boundaries (Numata Citation1969) (). There are currently 109 terrestrial mammal species in Japan (22 in Ryukyu and 24 in Hokkaido (MOE (Ministry of Environment) Citation2017); Table S7, S8).
The data from these sites can provide food range (dietary breadth) but only partial information on quantities consumed (Hockett and Haws Citation2003) (Table S9).
3. Methods
The methods of data search, abstraction, collation, filtering and statistical analysis are given in the Supplementary Text. The data is given here as the normalised frequency of presence per (number of sites) in each region by time period, where the periods are 16,000-5,300 c. cal. BP (H1/R1), 5,300-4,400 c. cal. BP (H2/R2), 4,400-2,800/2,300 c. cal. BP (H3/R3). See Table S1 for further detail.
4. Results
4.1 Fish
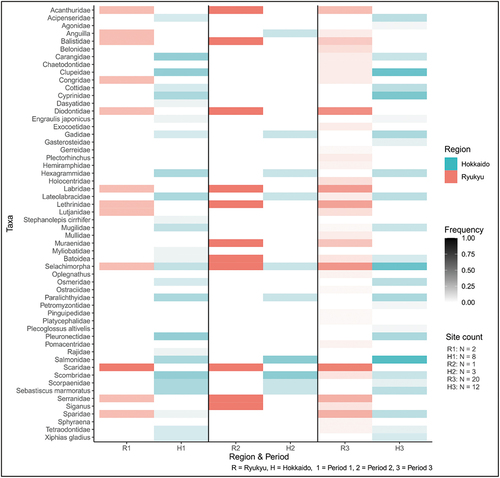
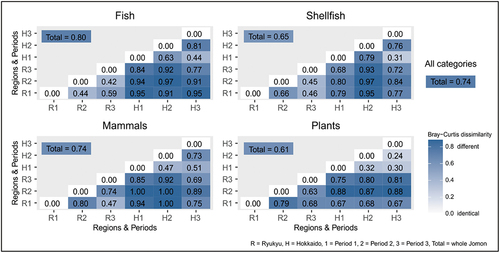
A total of 56 taxa were recorded (; Table S10) with the most common in Ryukyu being; porcupinefish (Diorontidae), parrotfish (Scaridae), and sharks (Selachimorpha) contrasting with those of Hokkaido (salmonids (Salmonidae), herrings (Clupeidae) and sharks). None of the common families/taxa of Ryukyu occurred in Hokkaido except within the sharks, Atlantic mako (Isurus oxyrinchus) and Japanese sea bass (Lateolabrax japonicus). The low overlap (20%) reflects the different marine-biogeographical areas – tropical waters around Ryukyu and temperate to Boreal waters around Hokkaido.
4.2 Shellfish
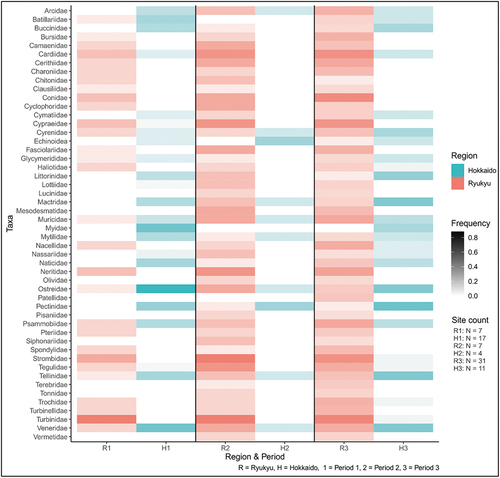
As there were 271 shellfish taxa (Table S10), shellfish were kept at the family level for this analysis (142 families, ). The three most common families in Ryukyu were turban snails (Turbinidae), cockles (Cardiidae) and cone shells (Conidae). In Hokkaido, the most common families were oysters (Ostreidae), venus clams (Veneridae), and scallops (Pectinidae). Even at the family level, the overlap in species was small (35%) with the main overlap in clams (Cyrenidae, two shared-species) and murex snails (Muricidae, one out of 38 species), and venus clams (Veneridae, two out of 38 species).
4.3 Mammals and birds
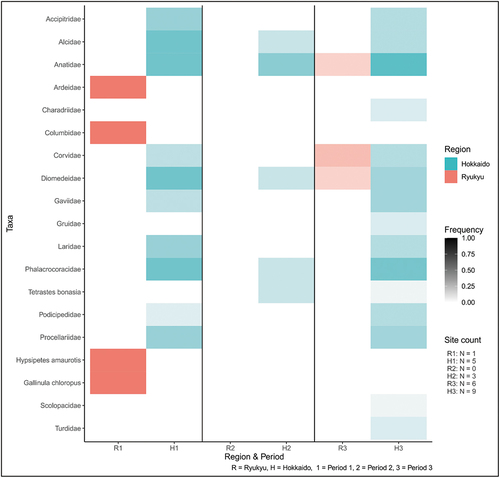
Of 25 taxa reported (; Table S10), dugong and whale/dolphin (Cetacea) were frequent in Ryukyu for marine mammals while in Hokkaido, northern fur seal and Japanese sea lion (Zalophus japonicus) were most common. Wild boar (Suidae), mouse (Muridae), and dog (Canis lupus subsp. familiaris) were most common in Ryukyu. The most common terrestrial taxa in Hokkaido, Hokkaido sika deer (Cervus nippon subsp. yesoensis), bear (Ursidae), and Ezo red fox (Vulpes vulpes subsp. schrencki), were absent from Ryukyu. Overall overlap is low (26%) and 8% if the synanthropic or semi-domesticated species are excluded.
Figure 5. Mammal taxa frequencies. * Include Capreolus and Cervidae (no genus or species mentioned) ** Include Sus scrofa subsp. scrofa in one site in each area.
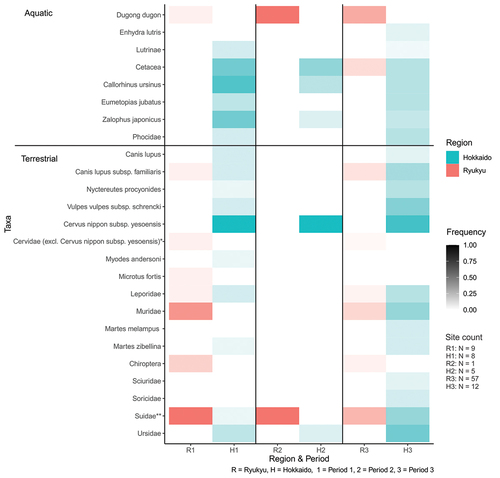
It is also apparent that the total number of taxa increased from Period 1 to 3 in Hokkaido but not in Ryukyu, although this may partly be due to the relative increase in the number of sites. Birds, at 20 taxa, are nearly as rich as mammals (; Table S10). The three most common bird families in Ryukyu were crows (Corvidae), ducks (Anatidae), and albatrosses (Diomedeidae), of which ducks and albatrosses were also common in Hokkaido with the addition of cormorants (Phalacrocoracidae).
4.4 Plants
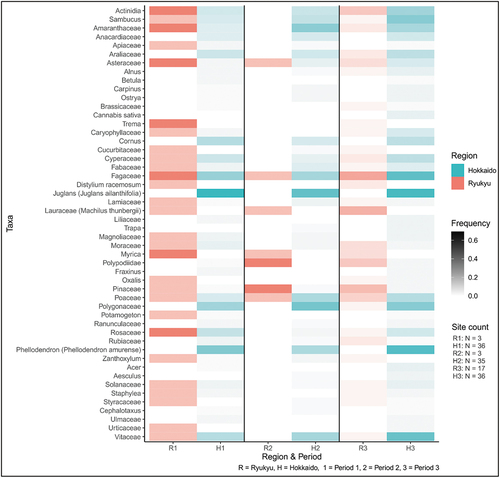
Out of 114 taxa, the top 50 plant taxa frequencies are shown in (Table S10). The most common plant taxa in Ryukyu throughout Jōmon were beech (Fagaceae), Japanese bay tree (Machilus thunbergii), and Chinese gooseberry (Actinidia rufa) whilst in Hokkaido, they were Japanese walnut (Juglans ailanthifolia), Amur cork tree (Phellodendron amurense), and beech. The beech in Ryukyu included chinkapin (Castanopsis sieboldii), Ryukyu oak (Quercus miyagii), black ridge oak (Q. phillyraeoides), and Japanese willowleaf oak (Quercus salicina) whereas those in Hokkaido were Japanese chestnut (Castanea crenata), mizunara (Q. crispula), daimyo oak (Q. dentata), and glandbearing oak (Q. serrata). Although 26 of the top 50 were shared at a family level between Ryukyu and Hokkaido, the only definite shared-species was elderberry (Sambucus racemosa subsp. sieboldiana).
4.5 Geographical differences
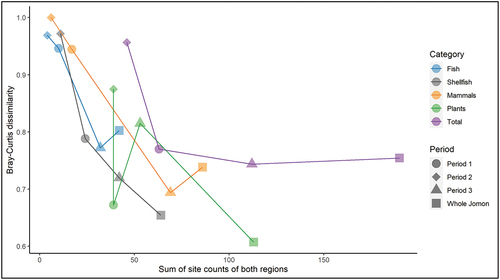
Overall, the taxa were 75% different between Ryukyu and Hokkaido (). The greatest difference observed was in fish, followed by mammals, shellfish, and plants. The partial dependence of Bray-Curtis dissimilarity values on the number of sites was assessed based on category () and periods (Figure S1). There was a general tendency for the similarity in taxa to increase as the total number of sites increased followed by flattening of the curve in all categories with the exceptions of plants and shellfish. The potential effect of low site numbers can also be seen in the high dissimilarity value for mammals in Period 2. The patterns are biogeographical with the greatest differences between terrestrial mammals (least well-dispersed and habitat-specific) followed by plants, fish, and shellfish. What is of archaeological significance is that even those groups best dispersed and with high levels of taxa with wide ecological ranges are still over 50% different.
5. Discussion
Preliminary analysis of this data largely supports observations for individual resource categories which were based on smaller numbers of sites. For fish, the high frequency of porcupine and parrotfish in Ryukyu was also reported by Takamiya et al. (Citation2016). For Hokkaido, boreal fish including North Pacific herring (Clupea pallasii), Pacific cod (Gadus macrocephalus), big-scaled redfin (Tribolodon hakonensis), and righteye flounder (Pleuronectidae) were common but also warm-current fish such as Japanese sea bass, flathead grey-mullet (Mugil cephalus), tuna (Thunnus), and Japanese amberjack (Seriola quinqueradiata) as recognised by Nishimoto (Citation1984). The most common fish family in Hokkaido, Salmonids, confirming studies by Okada (Citation1998) and Takase (Citation2019), increased between Periods 1 and 3, suggests fishing or trapping upstream or the use of different fishing tools (Uchimiya). The only shared-species (Japanese sea bass) found throughout the Jōmon, is a widely-distributed inshore and reef-fish requiring low technology for procurement. The seasonal migration of fish, such as Asian seabass, and flathead grey-mullet from inner bays to estuaries, Pacific herring to coastal shores in the spring, and the Salmonidae runs up rivers in the fall, makes these species easier to catch. The observation by Yamaura (Citation1998) of the increased importance of sea-mammal hunting in Hokkaido in the later Middle to Late Jōmon is supported by the number of sites rather than an increase in the diversity of procurement.
For Ryukyu as a whole, the most common shellfish are silver-mouthed turban (Turbo argyrostomus) and giant green turban (T. marmoratus), as observed from Okinawa by Kurozumi (Citation2014). In Hokkaido, Pacific oysters and Japanese carpet shells were most common as noted by Nishimoto (Citation1984). There are only two taxa for Ryukyu aquatic mammals in the database: dugong and whales/dolphins, but this could partly be due to low taxonomic resolution. Dugong, found in 60% of sites with mammal bones in Ryukyu, was an important protein source (Morimoto Citation2004). In Hokkaido, the most common types (whales/dolphins, fur seals and Japanese sea lions) were abundant in all three periods and also form major protein sources (Okada Citation1998; Takase Citation2019). Wild boar and mouse were the most commonly found terrestrial mammals in Ryukyu, as observed by Kawamura (Citation2007) and Takamiya et al. (Citation2016). Domestic dogs which along with wolves appeared in Ryukyu in the early Jōmon may be a misidentification and must be interpreted cautiously; however, dogs were hunting companions during the Jōmon, as is suggested by dog burials (Perri Citation2016; Yamada Citation1994). From the Initial Jōmon onwards, there are more than 110 cases of dog burials; most of them in the eastern half of north-central Honshu (Perri Citation2016). A likely dog burial was also found at a Final Jōmon site (Awayonagawabaru) in Okinawa (OPBE (Okinawa Prefectural Board of Education) Citation2019). The most common taxa in Hokkaido were Hokkaido sika deer as reported by Tsujino, Ishimaru, and Yumoto (Citation2010). It is also worth noting that wild boar bones were excavated from multiple Jōmon sites in Hokkaido although they are not regarded as native (Nishimoto Citation1984). Due to the low number of bones present only on the southern and eastern Pacific coast of Hokkaido, wild boars are thought to have been brought from Honshu as subadults (Nishimoto Citation1984). This is supported by the excavated infant wild boar-shaped clay figurines, suggesting that they had spiritual importance (Kobayashi Citation2014).
The most common trees in Ryukyu (chinkapin, oaks, and Japanese bay tree) and Hokkaido (Japanese walnut, Amur cork tree, and Japanese chestnut) confirmed previous studies (Crawford Citation2018; Takamiya et al. Citation2016; Yamada and Shibauchi Citation1997) and were staple plant-food sources and probably managed and/or cultivated (Kudo Citation2014). Some full-cultigens were found in Hokkaido but at low frequencies; some of which could be contaminations. Both soybeans and adzuki beans, which are found in the Late Jōmon in Kyushu and Honshu (Obata Citation2015), appear absent from Hokkaido and the Ryukyu Islands. Rice (Oryza sativa, three sites), barley (Hordeum vulgare, two sites), foxtail millet (Setaria italica, two sites), Proso millet (Panicum miliaceum, one site), Japanese barnyard millet (Echinochloa esculenta, two sites), and hemp (Cannabis, seven sites) are rare and so are unlikely to have made major dietary contributions. Jōmon cultivation of domesticated cereals has been much debated (Crawford Citation2008; Matsui and Kanehara Citation2006) but although introduced into some areas during the Late Jōmon, it is clear that they made up a minor component of regional Jōmon diets. Of more significance is the indigenous semi-domesticated or cultivated taxa (Actinidia, Fagaceae, Juglans ailanthifolia, Trapa, Aesculus and possibly Vitaceae; Table S11) which although a small proportion of the taxa (5%) are found at a large number of sites (Juglans 69.0% and Vitaceae 50.8% in Hokkaido) and so almost certainly were major dietary components (Fuller Citation2018). The only shared plant taxa, elderberry is particularly interesting as it has both nutritional benefits (a rich source of vitamin C, high antioxidants) and can be used for producing alcoholic drinks (Schmitzer et al. Citation2010; Senica et al. Citation2016).
The Bray-Curtis dissimilarity, scores for Periods 1 and 2 in both areas, need to be interpreted cautiously due to the low number of sites. However, comparing Period 1 with Period 3, we have dissimilarities in Ryukyu of 0.46–0.68 and 0.30–0.51 for Hokkaido. These are considerably lower than between the areas which vary from 0.67–0.95 for Period 1, to 0.69–0.81 for Period 3. This is fundamentally due to the biogeographical template and trophic level with the highest dissimilarities in mammals and fish, with more similarity in shellfish and plants. Due to the level of this analysis shared taxa, at 25% overall, are over-estimated and it is also likely that some environmental variation is hidden within the data such as the variation in the hard clams (Meretrix) with Asian hard clams (inland bays) represented in Periods 1 and 3 in Ryukyu whereas Lamarck’s hard clams are represented only in Period 3 (open sea). The alternative interpretation of differential procurement is unlikely due to similar size and nutritional values; however, these and all the shared shellfish species are intertidal/subtidal, with low procurement costs.
Even though we have here a comprehensive database, there remain potential biases including preservation. However, the unrivalled preservation conditions of most of these waterlogged-coastal sites and calcium carbonate in middens favour plant and bone preservation (Nakahashi and Nagai Citation1986). Another potential bias is the level of taxonomic resolution and differential sampling. Differential recovery is likely as pre-1990s sites rarely had flotation for plant remains (Barnes and Okita Citation1999); however, over 70% of the reports in the database were published after 1990, so this bias can be considered small.
5.1 Initial use for diet and resource-use comparison
The role of nutritional analyses of such data is to allow comparisons and relate this data to other data on both food-ways (e.g. pottery residue analysis) and human health (e.g. stable isotopes) in a heuristic manner. This approach makes several assumptions: firstly, that the taxa were used for food (as well as any other purposes). As we have only included taxa that excavators regarded as food-items and the data come from archaeological contexts, we can be reasonably confident all were eaten. Secondly, ideally, we need quantification of food-items, which is not available from the majority of sites and also poses many taphonomic challenges. We address this by using the commonality of taxa (frequency of occurrence) as an indicator that it was widely eaten and a staple food-type. Lastly, we are able to use modern nutritional values for the taxa because they were not, and never became domesticates, and our nutritional data derives from native/wild types.
The primary, and expected, finding of little overlap in potential food resources between the two areas raises the question; would the differences have led to nutritional or health differences or are these resources substitutable? This depends on the nutritional substitutability of food elements, and only an exemplar analysis is provided here, using only the taxa that achieve a frequency of occurrence of 0.6 or above (Table S12). From this data, we can see that the majority of calories in Ryukyu would have come from wild boar, dugong, some fish (e.g. sea bream), nuts and shellfish such as clams. There are several nutritional super-foods here (i.e. high in protein, fats/carbohydrates and omega acids) – including sea bream, chinquapin nuts, and sunset clams. Also notable is the number of foods that are both potentially toxic and have possible therapeutic/medical effects, especially on the cardiovascular system. This includes cone and cowrie gastropods, which contain taurine which regulates blood pressure, has antioxidant and anti-inflammatory properties, and has a protective effect against coronary heart disease (Wójcik et al. Citation2010). The high occurrence of Murex (Kaimurasaki/Tyrian purple) could also reflect its use for the purple dye, as known from textile contexts at the early Yayoi site of Yoshinogari, Kyushu (Maeda, Shimoyama, and Noda Citation1992). If it was also eaten, it has high omega-3, known to prevent vascular fat build-up (Salas et al. Citation2018). Overall, many of the Ryukyu taxa have high vitamins (D, B1, B12) and beneficial fatty acid ratios.
In Hokkaido, there is a more restricted list of staple food types with the main energy providers being Japanese walnut, sika deer, fur seals, salmon, herring and carp. Japanese walnut is also a nutritional super-food with high protein and fat as well as antibiotic properties (Juglone) (D’Angeli et al. Citation2021). This may have been important as the diet based on these foods is potentially low in fat/carbohydrate. Both areas have several staple foods with high omega (ω) 3 values, and beneficial fatty acid ratios (ω6/ω3) mostly from fish, and these would have had protective effects against coronary heart disease and a range of other disorders (Gómez Candela, Bermejo López, and Loria Kohen Citation2011). Vitamin B12-rich shellfish in both areas might have promoted child development (WHO-FAO (World Health Organization and Food and Agriculture Organization of the United Nations)). Except for the nut-trees, the resources are potentially available year-round although many would be easier to procure seasonally.
6. Conclusions
From this comprehensive (189 sites) and holistic (organism groups) data collation, there are conclusions at different scales of analysis.
1. In general, this dataset supports generalisations made from smaller datasets (e.g. Nishimoto Citation1984; Kurozumi Citation2014; Takamiya et al. Citation2016; Crawford and Bleed Citation1998). This reflects the commonality of the assemblages and provides increased confidence for inferences from smaller resource assemblages. Additionally, although only a small percentage of sites in Hokkaido contained imported domesticated cultivars, cultivated indigenous species were found in many sites suggesting a some but probably limited contribution to diets.
2. The data confirm that the utilized food-resources of all types were more different between the two areas than within either area over time. It is therefore not tenable to refer to a ‘Jōmon palaeoeconomy’ or single subsistence profile. This is driven principally by the biogeographical differences between the areas but probably modified by cultural preferences and seasonality. The question that remains to be resolved is what is the critical geographical scale at which this holds, and so can be used to define regional food-ways over time?
3. The few species that are shared between the two areas are either very widespread (Japanese sea bass), of cultural significance (domesticated dog, possibly elderberry), and/or introduced later in the Jōmon.
4. An initial comparison of the statistically most common food element on Jōmon sites from these two areas suggests a high dietary-breadth in both areas, but higher in Ryukyu than in Hokkaido, supporting higher biodiversity and food variety in a warmer climate. The main energy providers vary more in Ryukyu, but in both areas, shellfish have played an important role. Of particular note is the number of medicinal and therapeutic foods in Ryukyu, and the reliance on nuts for a balanced diet in Hokkaido. In both areas, the diets would have had high vitamin concentrations of B12 and advantageous fatty acid ratios, which would have been beneficial for both child development and the prevention of metabolic and cardiovascular diseases.
Whilst the Jomon societies of Hokkaido and the Ryukyu Islands shared a common culture to a large degree, it is clear that their subsistence bases were almost entirely different. Also, despite contacts, there was very little importation of subsistence-culture until the very late Jōmon. However, despite differences in species, the diets share many nutritional aspects – a very broad spectrum, a heavy emphasis on fish and seafood, and superfoods. We would argue this puts the nomological debate over agricultural activity into perspective shifting the debate onto the adaptive and societal values of Jōmon regional diets – an emerging issue in Japanese archaeology.
Data Availability
We will provide the data used in this paper to any bonafide requests to the authors.
Supplemental Material
Download PDF (1.9 MB)Acknowledgments
We are grateful for Rika Shinkai (RIHN Kyoto) for all her help and thank Hokkaido Archaeological Operations Center and Okinawa Prefectural Archaeological Center for assistance, as well as numerous museums.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00438243.2022.2089726
Additional information
Funding
Notes on contributors
Aya Komatsu
Aya Komatsu is a PhD Candidate at the Arctic University Museum of Norway, UiT – the Arctic University of Norway. Her research interest is nutrition and health. Currently, she is studying nutrition from a palaeoecological perspective.
Elisabeth J. Cooper
Elisabeth J. Cooper is the leader of the Northern Populations and Ecosystems research group at UiT – the Arctic University of Norway. She is a professor of plant ecology with special interest in responses of vegetation to climatic change.
Inger G. Alsos
Inger Alsos is the leader of the Norwegian Centre for Environmental DNA from Arctic Ecosystems Through Time (NEAT), UiT – Arctic University of Norway. Her research focus on past and likely future distribution of arctic and alpine vascular plants. She has combined genetic data (modern and ancient DNA), species distribution modelling, and fossil data to explore dispersal routes, colonization frequencies, and long-term genetic effects of climate change.
Antony G. Brown
Antony Brown is Research Professor at the University Museum, Arctic University of Norway, Tromsø, Norway and Professor in the Department of Geography and Environmental Science at the University of Southampton, UK. His research is broadly within geoarchaeology, palaeoecology and environmental archaeology. He is author of several books and over 200 research papers. His most recent projects have concerned the subsistence history and sustainability of coastal islands of Scotland, Ireland and Northern Norway, and the geoarchaeology of European agricultural terraces. He is particularly interested in applying nutritional thinking to archaeological datasets.
References
- Abe, C., C. Leipe, P.E. Tarasov, S. Müller, and M. Wagner. 2016. “Spatio-temporal Distribution of hunter–gatherer Archaeological Sites in the Hokkaido Region (Northern Japan): An Overview.” The Holocene 26 (10): 1627–1645. doi:10.1177/0959683616641745.
- Aikens, C.M., and T. Akazawa. 1996. ”The Pleistocene-Holocene Transition in Japan and Adjacent Northeast Asia.” In Humans at the End of the Ice Age. Interdisciplinary Contributions to Archaeology, edited by L.G. Straus, B.V. Eriksen, J.M. Erlandson, and D.R. Yesner, 109–128. Boston, MA: Springer. doi:10.1007/978-1-4613-1145-4_11.
- Barnes, G.L., and M. Okita. 1999. “Japanese Archaeology in the 1990s.” Journal of Archaeological Research 7 (4): 349–395. doi:10.1007/BF02446048.
- Crawford, G.W., and P. Bleed. 1998. “Scheduling and Sedentism in the Prehistory of Northern Japan.” In Seasonality and Sedentism: Archaeological Perspectives from Old and New World Sites, Peabody Museum Bulletins, edited by T.R. Rocek and O. Bar-Yosef, 109–128. Cambridge, MA: Peabody Museum of Archaeology and Ethnology, Harvard University.
- Crawford, G.W., and H. Takamiya. 2008. “Japanese Archipelago, Prehistoric Hunter-Fisher-Gatherers.” In Encyclopedia of Archaeology, edited by D.M. Pearsall, 637–641. New York: Academic Press.
- Crawford, G.W. 2008. “The Jomon in Early Agriculture Discourse: Issues Arising from Matsui, Kanehara and Pearson.” World Archaeology 40 (4): 445–465. doi:10.1080/00438240802451181.
- Crawford, G.W. 2018. “Palaeoethnobotanical Contributions to Human-Environment Interaction.” In Environmental Archaeology: Current Theoretical and Methodological Approaches, edited by E. Pişkin, A. Marciniak, and M. Bartkowiak, 155–180. Cham: Springer International Publishing.
- Crema, E.R., J. Habu, K. Kobayashi and M. Madella. 2016. “Summed Probability Distribution of 14C Dates Suggests Regional Divergences in the Population Dynamics of the Jomon Period in Eastern Japan.” PLOS ONE 11 (4): e0154809. doi:10.1371/journal.pone.0154809.
- Cummings, V. 2014. “Hunting and Gathering in a Farmers’ World.” In The Oxford Handbook of the Archaeology and Anthropology of Hunter-Gatherers, edited by V. Cummings, P. Jordan, and M. Zvelebil, 1–23. Oxford Handbooks Online. Oxford: Oxford University Press. doi:10.1093/oxfordhb/9780199551224.013.013.
- D’Angeli, F., G.A. Malfa, A. Garozzo, G. Li Volti, C. Genovese, A. Stivala, D. Nicolosi, et al. 2021. “Antimicrobial, Antioxidant, and Cytotoxic Activities of Juglans regia L. Pellicle Extract.” Antibiotics 10:159. doi:10.3390/antibiotics10020159.
- Fujikura, K., D. Lindsay, H. Kitazato, S. Nishida, Y. Shirayama, and J M. Schnur. 2010. “Marine Biodiversity in Japanese Waters.” PLOS ONE 5 (8): e11836. doi:10.1371/journal.pone.0011836.
- Fuller, D.Q. 2018. “Long and Attenuated: Comparative Trends in the Domestication of Tree Fruits.” Vegetation History and Archaeobotany 27 (1): 165–176. doi:10.1007/s00334-017-0659-2.
- GBIF.org, 2021. GBIF: The Global Biodiversity Information Facility Home Page. (accessed 4 April 2022). https://www.gbif.org/
- Gómez Candela, C., L.M. Bermejo López, and V. Loria Kohen. 2011. “Importance of a Balanced Omega 6/omega 3 Ratio for the Maintenance of Health: Nutritional Recommendations.” Nutr Hosp 26 (2): 323–329. doi:10.1590/S0212-16112011000200013.
- Gotanda, K., T. Nakagawa, P. Tarasov, J. Kitagawa, Y. Inoue, and Y. Yasuda. 2002. “Biome Classification from Japanese Pollen Data: Application to modern-day and Late Quaternary Samples.” Quaternary Science Reviews 21 (4–6): 647–657. doi:10.1016/S0277-3791(01)00046-4.
- Habu, J. 2004. Ancient Jomon of Japan. Cambridge: Cambridge University Press.
- Habu, J. 2015. “Mechanisms of long-term Culture Change and Human Impacts on the Environment: A Perspective from Historical Ecology, with Special Reference to the Early and Middle Jomon Periods of Prehistoric Japan.” The Quaternary Research 54 (5): 299–310. doi:10.4116/jaqua.54.299. In Japanese.
- Hockett, B., and J. Haws. 2003. “Nutritional Ecology and Diachronic Trends in Paleolithic Diet and Health.” Evolutionary Anthropology 12: 211–216. doi:10.1002/evan.10116.
- Hudson, M.J., I.R. Bausch, M. Robbeets, T. Li, J.A. White, and L. Gilaizeau. 2021. “Bronze Age Globalisation and Eurasian Impacts on Later Jōmon Social Change.” J World Prehist 34 (2): 121–158. doi:10.1007/s10963-021-09156-6.
- JBOSC (Japan Biodiversity Outlook Science Committee, The Ministry of the Environment). 2010. “Report of Comprehensive Assessment of Biodiversity in Japan.” In Global Biodiversity Strategy Office, Biodiversity Policy Division, Nature Conservation Bureau, 1–277. Tokyo, Japan: Ministry of the Environment.
- Kawamura, Y. 2007. “Last Glacial and Holocene Land Mammals of the Japanese Islands: Their Fauna, Extinction and Immigration.” The Quaternary Research 46 (3): 171–177. doi:10.4116/jaqua.46.171.
- Kitagawa, J., H. Kojima, T. Yoshida, and Y. Yasuda. 2018. “Adaptations of the Early Jomon People in Their Settlement Relocation to Climate Change around Lake Mikata, Central Japan.” Archaeological Research in Asia 16: 66–77. doi:10.1016/j.ara.2018.03.002.
- Kobayashi, M. 2014. ”Introduction of Collection Materials - Animal Clay Figurines.” SARANIP 53: 257. Hakodate: Hakodate City Museum.
- Koyama, S., and S. Sugito. 1984. “A Study of Jomon Population - Computer Simulation Analysis -.” Bulletin of the National Museum of Ethnology 9 (1): 1–39. doi:10.15021/00004436. In Japanese.
- Kubo, H., 2017. Problems on the Conservation of the Molluscan Diversity in Bay Area of Okinawa-jima Island. TAXA, Proceedings of the Japanese Society of Systematic Zoology. Ginowan City, Okinawa. 42, 16–21. doi:10.19004/taxa.42.0_16.
- Kudo, Y. 2014. New Perspectives on the Plant Use of Jomon People. Tokyo, Japan: Shinsensha. In Japanese.
- Kurozumi, T. 2014. “Environmental and Cultural Changes in the Okinawa Islands as Seen from the Remains of Shellfish”. In Environmental and Cultural Transition of Prehistoric and Protohistoric Ryukyu Islands, Collection of Research Papers on Empirical Research on the Transition of Environment and Culture in Prehistory and Protohistory of the Ryukyu Islands, edited by H. Takamiya and T. Shinzato, 55–70. Tokyo: Rokuichi Shobo. In Japanese.
- Kusaka, S., Y. Yamada, and M. Yoneda. 2018. “Ecological and Cultural Shifts of hunter-gatherers of the Jomon Period Paralleled with Environmental Changes.” Am J Phys Anthropol 167 (2): 377–388. doi:10.1002/ajpa.23638.
- MAFF (Ministry of Agriculture, Forestry and Fisheries). 2017. Current Status of Forest Resources (As of March 31, 2017. Forestry Agency. (accessed 1 April 2022). https://www.rinya.maff.go.jp/j/keikaku/genkyou/h29/index.html. In Japanese.
- Matsushima, Y. 2006. Jomon Transgression from Shellfish – the World at +2℃. Yokohama: Yurindo. In Japanese.
- Maeda, U., S. Shimoyama, and Noda. 1992. “Analysis of Die Excavated from Yoshinogari Site.” In Saga Prefecture Cultural Property Survey Report 113. Saga: Department of Cultural Properties, Saga Prefectural Board of Education. In Japanese.
- Matsui, A., and M. Kanehara. 2006. “The Question of Prehistoric Plant Husbandry during the Jomon Period in Japan.” World Archaeology 38 (2): 259–273. doi:10.1080/00438240600708295.
- MOE (Ministry of Environment). 2017. For the Inscription on the World Heritage List of Amami-Oshima Island, Tokunoshima Island, the Northern Part of Okinawa Island and Iriomote Island. Tokyo: Ministry of Environment, Government of Japan.
- Morimoto, I. 2004. “Preliminary Survey of Excavated Dugong Bones.” The Archaeological Study of Okinawa 2: 23–42. Nishihara Town, Okinawa: Okinawa Prefectural Archaeological Center.
- Morioka, Y., S. Varlamov, and Y. Miyazawa. 2019. “Role of Kuroshio Current in Fish Resource Variability off Southwest Japan.” Scientific Reports 9 (1): 17942. doi:10.1038/s41598-019-54432-3.
- Morley, J.J., L.E. Heusser, and T. Sarro. 1986. “Latest Pleistocene and Holocene Palaeoenvironment of Japan and Its Marginal Sea.” Palaeogeography, Palaeoclimatology, Palaeoecology 53 (2–4): 349–358. doi:10.1016/0031-0182(86)90068-4.
- Nakahashi, T., and M. Nagai. 1986. “Preservation of Human Bone in Prehistoric and Historic Sites of Western Japan.” Asian Perspectives 27: 15–27.
- Nakayama, S. 2015. “Domestication of the Soybean (Glycine Max) and Morphological Differentiation of Seeds in the Jomon Period.” Jpn. J. Histor. Bot. 23: 33–42. In Japanese.
- Nishimoto, T. 1984. “The Hunting and Fishing Activities of the Jōmon and the Epi-Jōmon Culture: An Analysis on the Faunal Remains.” Bulletin of the National Museum of Japanese History. 4: 1–15. In Japanese.
- Numata, M. 1969. “Ecological Background and Conservation of Japanese Islands.” Micronesica 5: 295–302.
- Obata, H., Miyaura, M., Nakano, K., 2020. ”Jomon pottery and maize weevils, Sitophilus zeamais, in Japan.” Journal of Archaeological Science: Reports 34: 102599. doi:10.1016/j.jasrep.2020.102599.
- Obata, H. 2015. The seed-sowing Jōmon people: Recent science overturns the origins of agriculture. Tokyo: Yoshikawa Kōbunkan. In Japanese.
- Ohe, F., M. Tajima, and R. Shinkai. 2016. “The Fish Remains from Hirozaki Shell Midden and the Paleoenvironment.” Zooarchaeology. 33: 17–33. In Japanese.
- Okada, A. 1998. “Maritime Adaptations in Hokkaido.” Arctic Anthropology 35 (1): 340–349.
- Ooi, N. 2016. “Vegetation History of Japan since the Last Glacial Based on Palynological Data.” Jpn. J. Histor. Bot. 25: 1–101.
- OPBE (Okinawa Prefectural Board of Education). 2019. Everyone’s Cultural Property Pictorial Book - Buried Cultural Properties Edition. Naha, Okinawa: Okinawa Prefectural Board of Education. In Japanese.
- Pearson, R. 2006. “Jomon Hot Spot: Increasing Sedentism in South-western Japan in the Incipient Jomon (14,000–9250 Cal. BC) and Earliest Jomon (9250–5300 Cal. BC) Periods.” World Archaeology 38 (2): 239–258. doi:10.1080/00438240600693976.
- Perri, A.R. 2016. “Hunting Dogs as Environmental Adaptations in Jōmon Japan.” Antiquity 90 (353): 1166–1180. doi:10.15184/aqy.2016.115.
- Robson, H.K., A. Lucquin, K. Gibbs, H. Saul, T. Tomoda, Y. Hirasawa, T. Yamahara, et al. 2020. “Walnuts, Salmon and Sika Deer: Exploring the Evolution and Diversification of Jōmon “Culinary” Traditions in Prehistoric Hokkaidō.” Journal of Anthropological Archaeology 60: 101225. doi:10.1016/j.jaa.2020.101225.
- Salas, S., K. Chakraborty, P.T. Sarada, and P. Vijayagopal. 2018. “Nutritional Composition of the Branched Murex Chicoreus Ramosus (Linnaeus, 1758) (Family: Muricidae).” Indian Journal of Fisheries 65 (4): 102–108. doi:10.21077/ijf.2018.65.4.79344-12.
- Schmitzer, V., R. Veberic, A. Slatnar, and F. Stampar. 2010. “Elderberry (Sambucus nigra L.) Wine: A Product Rich in Health Promoting Compounds.” J. Agric. Food Chem 58 (18): 10143–10146. doi:10.1021/jf102083s.
- Senica, M., F. Stampar, R. Veberic, and M. Mikulic-Petkovsek. 2016. “Processed Elderberry (Sambucus nigra L.) Products: A Beneficial or Harmful Food Alternative?” LWT - Food Science and Technology 72: 182–188. doi:10.1016/j.lwt.2016.04.056.
- Shimizu, Y. 2014. “Process of the Formation of Japanese Forest and Typification of Vegetation Zone - from an East Asian Viewpoint.” Chirigaku Kenkyu. 27: 19–75. In Japanese.
- Takahashi, R., T. Toizumi, and Y. Kojo. 1998. “Archaeological Studies of Japan: Current Studies of the Jomon Archaeology.” Journal of the Japanese Archaeological Association 5 (5): 47–72. doi:10.11215/nihonkokogaku1994.5.47
- Takahara, H., S. Sugita, S.P. Harrison, N. Miyoshi, Y. Morita, and T. Uchiyama. 2000. “Pollen-Based Reconstructions of Japanese Biomes at 0,6000 and 18,000 14C Yr BP.” Journal of Biogeography 27 (3): 665–683. doi:10.1046/j.1365-2699.2000.00432.x.
- Takamiya, H., M.J. Hudson, H. Yonenobu, T. Kurozumi, and K. Toizumi. 2016. “An Extraordinary Case in Human History: Prehistoric hunter-gatherer Adaptation to the Islands of the Central Ryukyus (Amami and Okinawa Archipelagos), Japan.” The Holocene 26 (3): 408–422. doi:10.1177/0959683615609752.
- Takase, K. 2019. “Long-term Marine Resource Use in Hokkaido, Northern Japan: New Insights into Sea Mammal Hunting and Fishing.” World Archaeology 51 (3): 408–428. doi:10.1080/00438243.2019.1699854.
- Tsujino, R., E. Ishimaru, and T. Yumoto. 2010. “Distribution Patterns of Five Mammals in the Jomon Period, Middle Edo Period, and the Present, in the Japanese Archipelago.” Mammal Study 35 (3): 179–189. doi:10.3106/041.035.0304.
- Umitsu, M. 1991. “Holocene Sea-Level Changes and Coastal Evolution in Japan.” The Quaternary Research 30 (3): 187–196. doi:10.4116/jaqua.30.187.
- WHO-FAO (World Health Organization and Food and Agriculture Organization of the United Nations). 2006. ”Evaluating the Public Health Significance of Micronutrient Malnutrition.” In Guidelines on Food Fortification with Micronutrients, 39–92. Geneva: WHO
- Wójcik, O.P., K.L. Koenig, A. Zeleniuch-Jacquotte, M. Costa, and Y. Chen. 2010. “The Potential Protective Effects of Taurine on Coronary Heart Disease.” Atherosclerosis 208 (1): 19–25. doi:10.1016/j.atherosclerosis.2009.06.002.
- Yamada, Y. 1994. “Dogs in the Jomon Period - with a Focus on Their Roles -.” Comparative Folklore Studies 9: 181–188. In Japanese.
- Yamada, G., and S. Shibauchi. 1997. “Nuts Excavated from Jomon Sites of Hokkaido - Especially about Chestnuts -.” Bulletin of the Historical Museum of Hokkaido. 25: 17–30. In Japanese.
- Yamano, H., K. Sugihara, and K. Nomura. 2011. “Rapid Poleward Range Expansion of Tropical Reef Corals in Response to Rising Sea Surface Temperatures.” Geophysical Research Letters 38 (4): L04601. doi:10.1029/2010GL046474.
- Yamaura, K. 1998. “The Sea Mammal Hunting Cultures of the Okhotsk Sea with Special Reference to Hokkaido Prehistory.” Arctic Anthropology 35: 321–334.
- Yamazaki, T. 2011. Molluscan Fauna of Akkeshi Marine Station: Field Science Center for Northern Biosphere, Hokkaido University: Part 1. Intertidal Areas. Hokkaido: Conchological Club of Northern Regions, Hokkaido University. In Japanese.